-
PDF
- Split View
-
Views
-
Cite
Cite
Anton Burlaka, Veronika Rozhkova, Artem Mykytuk, Liliia Babak, Natalia Kasap, Andriy Beznosenko, Laparoscopic S7 and S4 segmentectomy combined with transverse colon resection for synchronous metastatic colorectal cancer: a case report, Journal of Surgical Case Reports, Volume 2025, Issue 7, July 2025, rjaf476, https://doi.org/10.1093/jscr/rjaf476
Close - Share Icon Share
Abstract
Simultaneous laparoscopic resection of colorectal cancer (CRC) and synchronous liver metastases (SLM) remains a surgical challenge, especially when lesions involve posterosuperior liver segments. We present a case of a 42-year-old male with a transverse colon adenocarcinoma with SLM in segments 7 and 4. A simultaneous laparoscopic procedure was performed: anatomical liver resections in a modified left jack-knife position followed by transverse colectomy in the supine position. In-flow and out-flow blood control was achieved using intracorporeal Pringle manoeuvre and right hepatic vein tourniquet. The patient initiated a chemotherapy regimen with capecitabine and oxaliplatin (CAPOX) postoperatively and remained recurrence-free at 8 months. This case demonstrates the feasibility and safety of simultaneous laparoscopic resection for CRC with limited SLM involving challenging hepatic segments.
Introduction
Approximately 40% of patients with colorectal cancer (CRC) develop liver metastases throughout the course of the disease [1]. Surgery is the mainstay of treatment and R0 resection is highly recommended for both primary tumour and cites of distant metastases. The optimal approach to the multimodal management of CRC and synchronous liver metastases (SLM) remains controversial. Simultaneous resections may be considered for patients with good performance status and a limited liver tumour burden [2]. However, when both major liver resection and high-risk colorectal surgery are required, this combination should be avoided [3]. The data is scarce regarding the oncological and surgical effectiveness of simultaneous laparoscopic resections of a primary CRC with SLM. There are no unified guidelines for the laparoscopic ports’ placement and patient’s positioning when both middle and upper abdomen surgical stages are planned. Given the data from randomized trials on liver surgery, it can be argued that minimally invasive surgical approach can reduce surgical stress and postoperative complications, with blood loss comparable to open surgery [4, 5]. Therefore, laparoscopic simultaneous resections in patients with CRC and SLM may have an advantage over open surgery. We present a case report of laparoscopic resection of liver metastases allocated in S7 and S4 and simultaneous resection of the transverse colon.
Case presentation
A 42-year-old male patient presented with a 2-month history of melena, and a weight loss. The laboratory examination showed anaemia: red blood cells (RBC) – 3,9 × 109/L, Hb – 8.6 g/dl. The patient’s serum levels of carcinoembryonic antigen and carbohydrate antigen 19-9 were elevated—28.3 and 47.72 U/ml, respectively. Imaging of the abdomen using computed tomography (CT) and magnetic resonance techniques revealed a tumour measuring 25 × 35 mm in the transverse colon, along with two liver lesions located in segment 7 and segment 4 inferior, measuring 35 mm and 26 mm, respectively (Fig. 1).
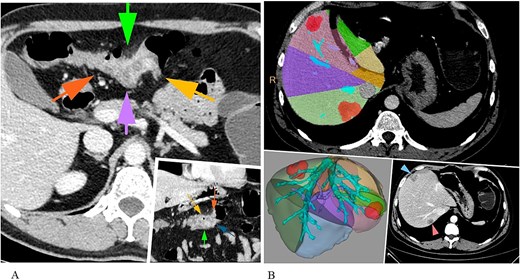
CT abdomen revealing a primary colon tumour (A) and liver metastases (S7, S4inf) (B).
Following evaluation, the multidisciplinary tumour board recommended proceeding upfront to surgery, with a planned simultaneous laparoscopic anatomical resection of liver segments 7 and 4 inferior, along with removal of the tumour in the transverse colon.
Liver surgery was conducted as a first surgical stage with application of Ligasure Maryland and ultrasonic cavitation device. The patient was positioned in the modified left jack-knife position (Fig. 2). The trocars were placed according to the technique described by Y. Okuda with 5 mm trocar in ninth intercostal space [6]. We used the intracorporeal intermittent Pringle manoeuvre technique according to Jian-Wei Huang for in-flow blood control [7]. Liver mobilization included transection of the right triangular, and falciform ligaments with patrial ‘piggy-back’ manoeuvre. 14 Fr Foley catheter was placed as a tourniquet on the right hepatic vein (RHV) to control the outflow (Video).
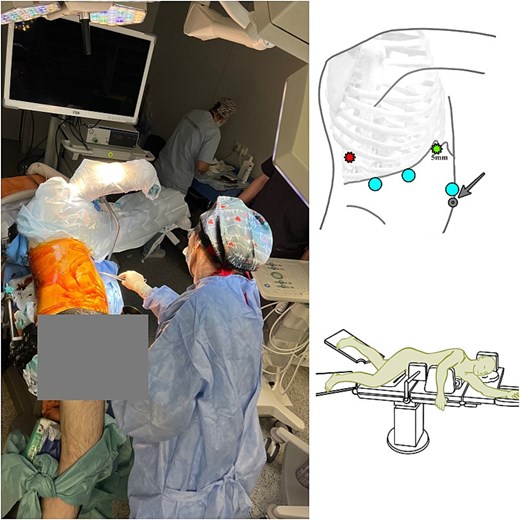
Patient's positioning on the operating table (modified left Jack-knife position) during liver resection. A 5-mm trocar was placed next to the xiphoid process, and the other two 12-mm trocars were placed at intervals of 6 cm next to the 5-mm trocar. The intercostal 5 mm trocar was placed through the ninth intercostal space on the right posterior axillary line.
The second (transverse colon) surgical stage demanded to reverse the patient’s position to supine. Two additional trocars were placed: 5 mm right paraumbilically and 5 mm infraumbilically (Fig. 3). A transverse colon resection was performed, followed by an extracorporeal, hand-sutured end-to-end colon anastomosis (Video).
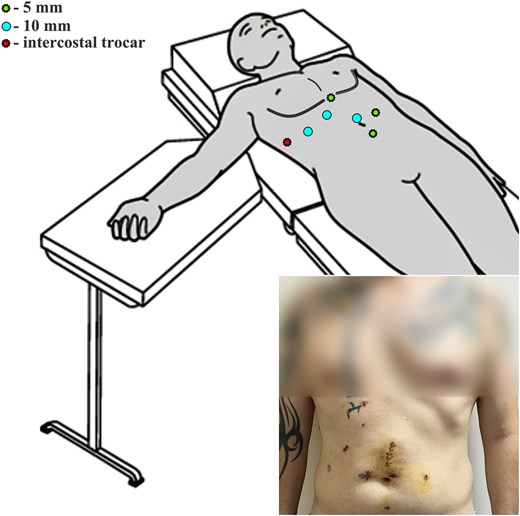
Graphic representation of the patient's positioning on the operating table (supine position) during colon resection and final view on the fifth postoperative day.
Total operative time was 345 min, with an estimated blood loss of ~150 ml, portal ischemia during Pringle manoeuvre was 28 min.
The postoperative adverse event was classified as Clavien-Dindo IIIA: the right-sided exudative pleuritis was treated with a percutaneous drainage of the right pleural sinus on fourth postoperative day, yielding up to 300 ml of serous fluid. Empirical antibiotic therapy with amoxicillin/clavulanic acid (875 mg twice daily for 5 days orally) was initiated. The patient required a transfusion of two red blood cells units due to anaemia (Hb – 6.4 g/dl). By the time of discharge, the Hb level had increased to 10.8 g/dl.
Histopathological examination of the resected liver and colon specimens confirmed moderately differentiated adenocarcinoma: pT3 pN0 pM1. Molecular genetic analysis revealed a G12X-G13D mutation in exon 2 of the NRAS gene with stable MSS/MSI, KRAS, BRAF—wild type. Three weeks post-discharge, systemic chemotherapy was initiated using the CAPOX regimen. At 3 and 8 months postoperatively, CT showed no recurrence or metastases.
Discussion
A laparoscopic approach to liver surgery minimizes surgical trauma, leading to reduced postoperative pain, faster recovery, and a shorter hospital stay [5, 6]. However, simultaneous laparoscopic resection in CRC patients with SLM is a technically demanding procedure. Metastatic lesions allocated in posterosuperior segments or right ‘venous liver core’ have been identified as challenging by laparoscopic surgeons' societies [8]. Bleeding and failure to progress are the two primary reasons for conversion to open surgery in these cases, largely due to the limited angles of attack and the complex vascular anatomy [9]. The experience of open surgery is of great importance for understanding the basic principles of safety, which subsequently allows liver resections to be performed through a minimally invasive laparoscopic surgical approach [10]. In open surgery we apply the basic principles of the gentle right liver lobe mobilization and vascular tourniquets to control both blood inflow and outflow to minimize the bleeding [11, 12]. It is well known that laparoscopic surgery reduces parenchymal blood loss through carboperitoneum, which at the same time, jeopardizes patient’s safety in case of main hepatic veins injuries [13, 14]. That is why we recommended controlling the common trunk of RHV with tourniquet during the laparoscopic hepatic transection of S7.
In conclusion, laparoscopic anatomical resection of the S7 and S4 with simultaneous resection of the colon transversum can be safely performed through seven trocars with a change in patient’s positioning before the colon resection. Intracorporeal control of blood in- and outflow can reduce the risk of bleeding during the S7 resection.
Conflict of interest statement
The authors declare there is no conflict of interest.
Funding
None declared.
Patient consent
Written informed consent for data processing was obtained from the patient.
References
- chemotherapy regimen
- colorectal cancer
- adenocarcinoma
- hepatic resection
- laparoscopy
- mastectomy, segmental
- safety
- supine position
- surgical procedures, operative
- tourniquets
- liver
- liver metastases
- capecitabine
- oxaliplatin
- pringle maneuver
- colectomy, transverse
- colorectal cancer metastatic
- transverse colon
- right hepatic vein
- liver excision, laparoscopic
- capox regimen
- fluid flow



