-
PDF
- Split View
-
Views
-
Cite
Cite
Yingtong Gao, Elisabeth Savonitto, Shuyin Liang, Alison Wallace, Efficacy of negative pressure wound therapy blowhole placement in alleviating severe subcutaneous emphysema and associated transient blindness following video-assisted thorascopic surgery: a case series, Journal of Surgical Case Reports, Volume 2025, Issue 7, July 2025, rjaf449, https://doi.org/10.1093/jscr/rjaf449
Close - Share Icon Share
Abstract
Video-assisted thorascopic surgery (VATS) lobectomy is widely used for treating lung cancer, but severe subcutaneous emphysema can be a rare complication, leading to distressing symptoms such as pain, temporary blindness, and voice changes. Traditional management strategies often require prolonged treatment, and blowhole incisions can result in infection and discomfort. This case series explores the use of negative pressure wound therapy (NPWT) applied via a unilateral blowhole incision to treat severe subcutaneous emphysema post-VATS lobectomy. In three cases, NPWT facilitated rapid resolution of pain, restored vision, and resolved voice changes within 8 h. No infection-related complications occurred. This approach not only accelerates recovery and alleviates patient discomfort but also reduces the burden on caregivers and healthcare teams. NPWT should be considered a valuable addition to the management of severe subcutaneous emphysema, improving patient outcomes and enhancing postoperative care.
Introduction
Video-assisted thorascopic surgery (VATS) lobectomy has emerged as an effective minimally invasive surgical technique for the treatment of lung cancer and other thoracic conditions. It is currently established internationally as the approach of choice for surgical resection of early-stage lung cancers. VATS offers multiple advantages over traditional thoracotomy, including decreased postoperative pain, shorter hospital stay, and fewer postoperative complications [1].
However, one of the rare complications following VATS lobectomy is the development of severe subcutaneous emphysema [2], which has also been reported in instances of trauma, pneumothorax, infection, malignancy, and other surgical procedures [3]. Although subcutaneous emphysema rarely causes any physiologic problems [3], it is often distressing to patients, their families, and their healthcare providers. This condition arises from the escape of air into the subcutaneous spaces of the chest wall, from where it may spread into the surrounding soft tissues [4]. This can lead to tense subcutaneous distension causing pressure-related symptoms, particularly around the face and neck, resulting in cosmetic deformities. In some severe cases, patients may experience a frightening consequence: temporary blindness due to the pressure of accumulated air causing the eyelids to swell and shut [3]. Other complications include pain due to tissue tension and nerve compression, as well as voice alterations [3].
Current management strategies for severe subcutaneous emphysema post-VATS are limited, focusing primarily on observation and oxygen therapy [3], and the use of blowhole incision(s) [5]. However, observation alone, in which soft tissues gradually reabsorb air, and conventional blowhole incisions, which rely on passive air drainage, can both require prolonged treatment for complete resolution of significant subcutaneous emphysema. For cases of severe subcutaneous emphysema, a more rapid method of removing subcutaneous air is needed. Moreover, blowhole incisions have certain drawbacks. They are associated with nosocomial wound infection since the wound needs to be kept open for sufficient air drainage [6]. Furthermore, regular dressing changes are needed, which can lead to patient discomfort [6]. Other methods for managing subcutaneous emphysema have also been described, including subcutaneous drain insertion and increasing suction on in situ chest tubes, each of which carries its own limitations [7]. However, none of these techniques have proven consistently effective and there is no consensus on the most favorable approach [7].
Negative pressure wound therapy (NPWT) is a widely used modality in wound management based on the effects of negative pressure suction on wound healing. One proposed mechanism of action of NPWT involves drawing fluids out of wounds, thereby reducing edema [8]. By the same principle, NPWT can be applied to blowhole incisions to extract trapped air and facilitate more rapid resolution of subcutaneous emphysema. Sciortino et al. [9] first described the application of NPWT for treating severe subcutaneous emphysema in 2009. Several case reports have since demonstrated its potential as a treatment, but it is still not widely used [6]. Our experience supports the use of NPWT in conjunction with a unilateral blowhole incision for more effective resolution of extensive subcutaneous emphysema and its often distressing consequences, including temporary blindness, pain, and voice changes.
Case series
We present our initial single-surgeon experience using NPWT in combination with a unilateral blowhole incision to manage severe subcutaneous emphysema resulting in temporary blindness in three patients following VATS lobectomy for lung cancer. The consistent and rapid resolution of symptoms observed in these cases led us to adopt this approach as standard practice for managing this distressing complication (Fig. 1).
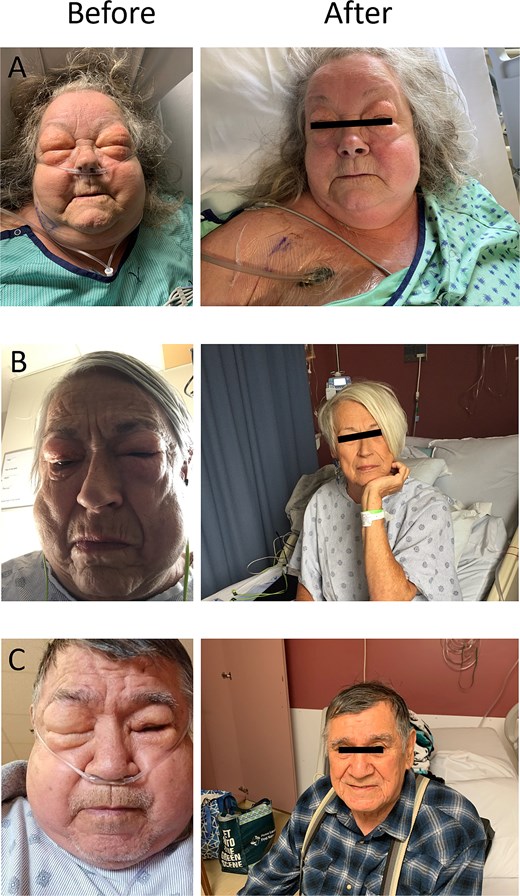
Before and after images of three patients who developed severe subcutaneous emphysema causing temporary blindness following VATS lobectomy. The “before” images demonstrate significant facial swelling and eye closure due to the accumulation of subcutaneous air. The “after” images show resolution of subcutaneous emphysema and restored vision within 12 h following the application of NPWT via a unilateral blowhole incision on the anterior chest wall. (A) 8 h after initiation of therapy. (B) 24 h after initiation of therapy. (C) 72 h after initiation of therapy.
Patient characteristics
A 69-year-old former smoker with a 36 pack-year smoking history who quit 7 years prior underwent a VATS right lower lobectomy. Pulmonary function tests showed a forced expiratory volume in one second (FEV₁) of 1.45 l (91% predicted), a FEV₁/forced vital capacity (FVC) of 65%, and a diffusion capacity 74% predicted. Final pathology revealed adenocarcinoma staged as pT1bN1 (Stage IIB) (Table 1).
| Patient A . | Patient B . | Patient C . | |
|---|---|---|---|
| Age | 69 | 73 | 78 |
| Smoking status | Former smoker Quit 7 years ago 36 pack/years | Current smoker 48 pack/years | Former smoker Quit 5 years ago 54 pack/years |
| BMI | 44 kg/m2 | 25.1 kg/m2 | 27.1 kg/m2 |
| FEV1 | 1.45 L or 91% predicted | 1.73 L or 74% predicted | 2.33 L or 90% predicted |
| FEV1/FVC | 65 | 58 | 54 |
| DLCO | 74% predicted | 64% predicted | 47% predicted |
| Surgical procedure | VATS right lower lobectomy | VATS left upper lobectomy | VATS right lower lobectomy |
| Final pathology | pT1bN1 Adenocarcinoma | pT2aN0 Squamous cell carcinoma | pT4N0 Squamous cell carcinoma |
| Patient A . | Patient B . | Patient C . | |
|---|---|---|---|
| Age | 69 | 73 | 78 |
| Smoking status | Former smoker Quit 7 years ago 36 pack/years | Current smoker 48 pack/years | Former smoker Quit 5 years ago 54 pack/years |
| BMI | 44 kg/m2 | 25.1 kg/m2 | 27.1 kg/m2 |
| FEV1 | 1.45 L or 91% predicted | 1.73 L or 74% predicted | 2.33 L or 90% predicted |
| FEV1/FVC | 65 | 58 | 54 |
| DLCO | 74% predicted | 64% predicted | 47% predicted |
| Surgical procedure | VATS right lower lobectomy | VATS left upper lobectomy | VATS right lower lobectomy |
| Final pathology | pT1bN1 Adenocarcinoma | pT2aN0 Squamous cell carcinoma | pT4N0 Squamous cell carcinoma |
Sex is not included to help conceal the identity of the patients.
| Patient A . | Patient B . | Patient C . | |
|---|---|---|---|
| Age | 69 | 73 | 78 |
| Smoking status | Former smoker Quit 7 years ago 36 pack/years | Current smoker 48 pack/years | Former smoker Quit 5 years ago 54 pack/years |
| BMI | 44 kg/m2 | 25.1 kg/m2 | 27.1 kg/m2 |
| FEV1 | 1.45 L or 91% predicted | 1.73 L or 74% predicted | 2.33 L or 90% predicted |
| FEV1/FVC | 65 | 58 | 54 |
| DLCO | 74% predicted | 64% predicted | 47% predicted |
| Surgical procedure | VATS right lower lobectomy | VATS left upper lobectomy | VATS right lower lobectomy |
| Final pathology | pT1bN1 Adenocarcinoma | pT2aN0 Squamous cell carcinoma | pT4N0 Squamous cell carcinoma |
| Patient A . | Patient B . | Patient C . | |
|---|---|---|---|
| Age | 69 | 73 | 78 |
| Smoking status | Former smoker Quit 7 years ago 36 pack/years | Current smoker 48 pack/years | Former smoker Quit 5 years ago 54 pack/years |
| BMI | 44 kg/m2 | 25.1 kg/m2 | 27.1 kg/m2 |
| FEV1 | 1.45 L or 91% predicted | 1.73 L or 74% predicted | 2.33 L or 90% predicted |
| FEV1/FVC | 65 | 58 | 54 |
| DLCO | 74% predicted | 64% predicted | 47% predicted |
| Surgical procedure | VATS right lower lobectomy | VATS left upper lobectomy | VATS right lower lobectomy |
| Final pathology | pT1bN1 Adenocarcinoma | pT2aN0 Squamous cell carcinoma | pT4N0 Squamous cell carcinoma |
Sex is not included to help conceal the identity of the patients.
A 73-year-old current smoker with a 48 pack-year smoking history underwent a VATS left upper lobectomy. Pulmonary function tests revealed a FEV₁ of 1.73 l (74% predicted), a FEV₁/FVC of 58%, and a diffusion capacity 64% predicted. Pathology confirmed squamous cell carcinoma staged as pT2aN0 (Stage IB) (Table 1).
A 78-year-old former smoker with a 54 pack-year smoking history who quit 5 years prior underwent a VATS right lower lobectomy. Pulmonary function tests indicated a FEV₁ of 2.33 l (90% predicted), a FEV₁/FVC of 54%, and a diffusion capacity 47% predicted. Pathology revealed squamous cell carcinoma staged as pT4N0 (Stage IIIA) (Table 1).
Table 2 describes the air leak management strategy including chest tube and chest drainage system and whether or not a pneumothorax was present on the immediate postoperative chest X-ray. Furthermore, the time course for development of severe subcutaneous emphysema, pain score at onset, and time for temporary blindness to resolve after intervention are outlined.
| Patient A . | Patient B . | Patient C . | |
|---|---|---|---|
| Management strategy | Single 24 F chest tube to analog chest drainage system | Single 24 F chest tube analog chest drainage system | Single 24 F chest tube to analog chest drainage system |
| Postop air leak status | Present—3/5 | Present—1/5 | Present—2/5 |
| Pneumothorax on postop chest X-ray | No pneumothorax | No pneumothorax | 2–3 mm apical pneumothorax |
| Suspected cause of subcutaneous emphysema | Kinked chest tube | Unknown | Unknown |
| Time of subcutaneous emphysema onset | POD 0–6 h postop | POD 6 | POD 7 |
| Initial pain score | 10/10 | 8/10 | 6/10 |
| Time temporary blindness resolved after intervention | 6 h | 8 h | 4 h |
| Patient A . | Patient B . | Patient C . | |
|---|---|---|---|
| Management strategy | Single 24 F chest tube to analog chest drainage system | Single 24 F chest tube analog chest drainage system | Single 24 F chest tube to analog chest drainage system |
| Postop air leak status | Present—3/5 | Present—1/5 | Present—2/5 |
| Pneumothorax on postop chest X-ray | No pneumothorax | No pneumothorax | 2–3 mm apical pneumothorax |
| Suspected cause of subcutaneous emphysema | Kinked chest tube | Unknown | Unknown |
| Time of subcutaneous emphysema onset | POD 0–6 h postop | POD 6 | POD 7 |
| Initial pain score | 10/10 | 8/10 | 6/10 |
| Time temporary blindness resolved after intervention | 6 h | 8 h | 4 h |
| Patient A . | Patient B . | Patient C . | |
|---|---|---|---|
| Management strategy | Single 24 F chest tube to analog chest drainage system | Single 24 F chest tube analog chest drainage system | Single 24 F chest tube to analog chest drainage system |
| Postop air leak status | Present—3/5 | Present—1/5 | Present—2/5 |
| Pneumothorax on postop chest X-ray | No pneumothorax | No pneumothorax | 2–3 mm apical pneumothorax |
| Suspected cause of subcutaneous emphysema | Kinked chest tube | Unknown | Unknown |
| Time of subcutaneous emphysema onset | POD 0–6 h postop | POD 6 | POD 7 |
| Initial pain score | 10/10 | 8/10 | 6/10 |
| Time temporary blindness resolved after intervention | 6 h | 8 h | 4 h |
| Patient A . | Patient B . | Patient C . | |
|---|---|---|---|
| Management strategy | Single 24 F chest tube to analog chest drainage system | Single 24 F chest tube analog chest drainage system | Single 24 F chest tube to analog chest drainage system |
| Postop air leak status | Present—3/5 | Present—1/5 | Present—2/5 |
| Pneumothorax on postop chest X-ray | No pneumothorax | No pneumothorax | 2–3 mm apical pneumothorax |
| Suspected cause of subcutaneous emphysema | Kinked chest tube | Unknown | Unknown |
| Time of subcutaneous emphysema onset | POD 0–6 h postop | POD 6 | POD 7 |
| Initial pain score | 10/10 | 8/10 | 6/10 |
| Time temporary blindness resolved after intervention | 6 h | 8 h | 4 h |
Surgical technique and postoperative course
To minimize the risk of air leaks during VATS lobectomy, we employ standard techniques [10, 11]. Despite this, air leaks remain the most common postoperative complication following VATS lobectomy at our institution, occurring in ~22% of cases (based on institutional data from 2020 to 2023). At the end of each procedure, a single 24 F chest tube is placed intraoperatively, connected to a chest drainage system and set to −20 cm H₂O suction, and a chest X-ray is performed in the recovery room (Table 2).
Severe subcutaneous emphysema leading to temporary blindness developed between 6 h and 7 days postoperatively (Table 2). In each case, the chest tube and collection system were assessed immediately to ensure proper function and replaced if necessary. Bronchoscopy was performed in all cases to rule out a bronchopleural fistula. NPWT was applied via a unilateral blowhole incision on the anterior chest wall on the same side as the lobectomy to facilitate rapid resolution of subcutaneous emphysema causing temporary blindness, pain, and voice changes.
The procedure was performed at the bedside under local anesthesia. A 4 cm incision was made on the anterior chest wall, ~2 cm below the clavicle on the operative side (Fig. 2). The incision was deepened down to the fascia using blunt dissection, and hemostasis was assured. One piece of black foam was inserted in the incision, which was then sealed airtight. Essentially, a traditional blowhole is created as previously described [5], and then NPWT was applied. The NPWT device was set to −125 mmHg of negative pressure, and dressing changes were scheduled every 3 days. Patients and family members were encouraged to perform gentle manual massage of the subcutaneous air toward to NPWT site to speed resolution. At the time of discharge, patients were transitioned to daily packing and dressing changes until complete healing.
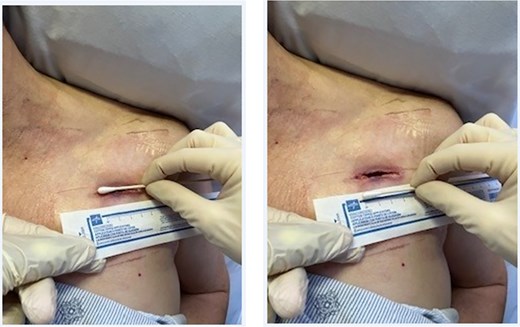
Four centimeter blowhole incision on the anterior chest wall to which the NPWT was applied.
Discussion
This case series demonstrates the effectiveness of NPWT applied via a unilateral blowhole incision for the management of severe subcutaneous emphysema causing temporary blindness, pain, and voice changes following VATS lobectomy. Air leaks remain a common postoperative complication, with the reported incidences ranging from 8% to 26% [10, 11]. Despite risk assessment, meticulous surgical techniques and appropriate chest tube management in rare cases subcutaneous emphysema can still develop causing distressing symptoms such as temporary blindness, pain, and voice changes requiring efficient and effective management.
In all cases, pain resolved almost immediately, while vision improved and voice changes resolved within 8 h of NPWT application (Table 2), providing rapid symptom relief and reassurance to patients, families, and healthcare providers. Based on our effective and consistent initial experience using a standardized approach to manage post-lobectomy subcutaneous emphysema, we have adopted this technique as standard practice for patients experiencing distressing symptoms such as temporary blindness or pain. Our center performs over 400 VATS lung resections annually. Since 2023, we have treated 10 patients using this protocol: a unilateral blowhole incision is made, and NPWT is applied directly to the incision at −125 mmHg. NPWT dressings are changed every 3 days. When the patient is ready for discharge, NPWT is discontinued and the wound is managed with daily packing until no longer clinically necessary. Notably, no infection-related complications have been observed to date.
By accelerating recovery, reducing discomfort, and lowering infection risk compared to traditional blowhole incisions, this approach not only benefits patients but also alleviates distress for their families and healthcare providers. Additionally, restoring vision helps mitigate the risk of postoperative delirium related to sensory impairment in elderly patients [12].
Given its success in the postoperative setting, the use of NPWT via a unilateral blowhole incision represents a valuable addition to management strategies for severe subcutaneous emphysema, with the potential to improve patient outcomes and reduce the burden on caregivers and the healthcare team.
Author contributions
All authors read and approved the final manuscript.
Conflict of interest statement
AW holds the position of member of the board of reviewers for the journal and is excluded from the peer review process and making decisions for the manuscript. AW has received honorariums from AstraZeneca, BristolMyersSquibb, and Merck.
Funding
No funding source was needed.
Data availability
Not applicable.
Consent
Written informed consent was obtained from all patients included in this case series, specifically permitting the publication of their facial photographs.
Guarantor
The corresponding author is the guarantor.



