-
PDF
- Split View
-
Views
-
Cite
Cite
Jenny Bui, Timothy Chalom, Saul D Nathanson, Theresa L Schwartz, Karen Hunt, Wamidh Alkhoory, Zhengfan Xu, Invasive ductal carcinoma at the site of a cosmetic nipple piercing, Journal of Surgical Case Reports, Volume 2025, Issue 3, March 2025, rjaf132, https://doi.org/10.1093/jscr/rjaf132
Close - Share Icon Share
Abstract
We report a young female patient diagnosed with an invasive ductal carcinoma at the site of a prior cosmetic nipple piercing. She had no significant familial, genetic, or other carcinogenic risk factors to account for her presentation. A review of the literature confirms that trauma can occasionally be associated with invasive breast cancer, but such a connection has not previously been related to nipple piercing procedures.
Introduction
Nipple piercing for display of jewelry has gained popularity as a form of body modification. The procedure of piercing the nipple while usually safe, can result in acute and chronic complications [1].
The average lifetime risk of developing breast cancer (BC) is about one-in-eight (12%), but it is 0.03% (30 cases per 100 000) in women in their thirties [1, 2]. The BC incidence is higher in young women with pathogenic mutations of BC associated genes, such as BRCA1 and BRCA2, and in women treated with mantle radiation for Hodgkin’s disease [2, 3].
Repetitive trauma that creates a breach in epithelial integrity stimulates wound healing, a precise, self-limiting series of biochemical and cellular events orchestrated by activation of specific signaling pathways [4]. Interruption of these pathways delays healing and leads to chronic wounds [4]. Aberrant initiation of wound healing may produce cell behavior that promotes carcinogenesis [4]. Chronic skin wounds can produce a malignant tumor in the scar tissue of burns or other traumatic injuries to the skin [5].
We present a case of a 33-year-old woman diagnosed with invasive ductal carcinoma (IDC) in her left breast, localized to the site of a prior nipple piercing, and review the possible association with the trauma of this procedure and carcinogenesis.
Case report
An otherwise healthy 33-year-old G2P2 African American female presented with a palpable left breast mass. She had no personal or family history of breast, ovarian, or other cancers, and no prior mantle irradiation or chronic use of exogenous hormones. She had a history of bilateral complication-free nipple piercings 12 years prior to presentation.
On examination, a superficial, visible, 1.1 cm mass was palpated within two centimeters of the left nipple associated with the scar of the previous nipple piercing. Mammogram failed to identify the mass, and an ultrasound showed no sonographic correlate to the palpable finding. An epidermoid cyst associated with the previous nipple piercing was diagnosed. A surgical excision revealed IDC, grade 2, ER/PR positive, HER2/neu negative with tumor present at the margins.
Magnetic resonance breast imaging revealed T2 enhancement surrounding the excisional biopsy cavity in the subareolar breast (Fig. 1a and b) and no suspicious internal mammary or axillary lymphadenopathy. Genetic testing did not identify any known pathogenic mutations associated with BC.
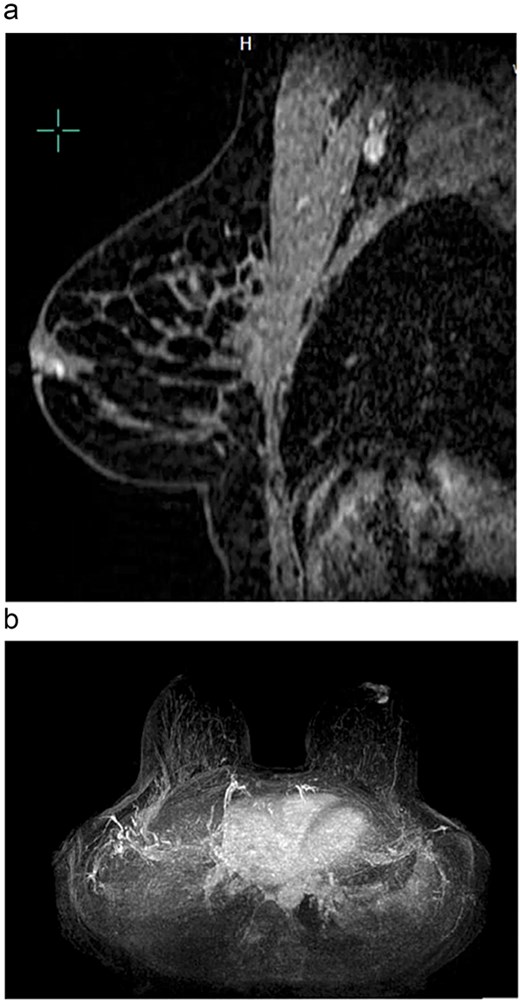
(a) Sagittal view of left breast retroareolar lesion. Enhancement appears to align in a duct in the retroareolar breast. (b) Axial view of left breast retroareolar lesion. Enhancement appears to align in a duct in the retroareolar breast.
A complete left central partial mastectomy with sentinel lymph node biopsy was performed. The lumpectomy specimen revealed IDC, infiltrating into the deep dermis of the overlying nipple (Fig. 2). Pathological stage was IA T1cN0M0 IDC. Adjuvant chemotherapy was not indicated because of a low Oncotype DX score. She completed adjuvant left whole-breast radiation but declined adjuvant endocrine therapy.
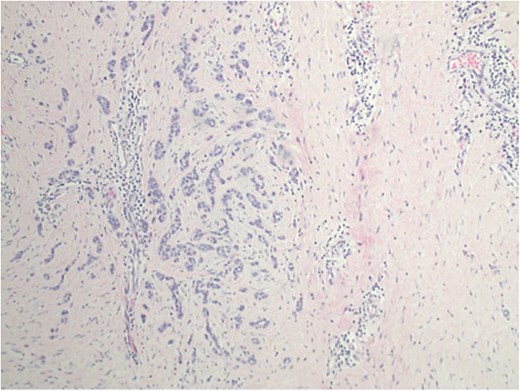
Invasive ductal carcinoma infiltrating into the deep dermis of the overlaying nipple.
Discussion
While basal cell carcinomas have been reported at the site of a nasal and ear piercing [6, 7], only one case of Paget’s disease in the site of nipple piercing has been reported [8]. The procedure of actively producing a fistula through the base of the nipple while prompting wound healing and scarring is not a known cause of IDC. However, maintenance of the fistula by jewelry provides a pathway for migration of micro-organisms and likely stimulates chronic inflammation. Long-term complications including scarring, nipple duct obstruction, and recurrent abscesses can lead to chronic local problems around the nipple [1].
The wound microenvironment induced by trauma has been reported to promote carcinogenesis in mice, zebra fish and human patients [4]. Deliberate traumatic wounds adjacent to growing syngeneic BC produced a significantly higher incidence and larger size of tumor growth [8]. Repeated wounding led to an increased incidence of melanoma in a Ras G12V-driven melanoma model in zebra fish [9, 10]. A relationship between wound healing and cancer progression can also be inferred from Marjolin ulcers of the skin, seen in pressure sores, chronic venous ulcers, fistulas, and chronic wounds [5]. Chronic and repeated exposure to heat from burning coals in Kangri containers placed next to the skin during cold seasons in Kashmir, India was observed to produce squamous cell carcinoma [11, 12].
Chronic inflammatory responses common to both cancer growth and wound healing have been reported to create conditions that support cellular proliferation and mutation, potentially leading to cancer development [13]. These activities require the interaction of various cell types, aided by growth factor and cytokine signaling networks. Trauma-induced inflammation triggers the release of chemokines and cytokines from the damaged tissue and surrounding stroma ultimately fostering a pro-tumorigenic environment [14].
Breast cancer in young women can sometimes be traced to a familial cancer history, associated, with or without pathogenic mutations in BC associated genes. Another cause of BC in young women is exposure to mantle radiation for conditions like Hodgkin’s lymphoma during adolescence or early adulthood [2, 3].
The absence of other significant risk factors such as genetic mutations, previous mantle radiation, or a family history of BC raises the possibility that the nipple piercing is a more likely contributing factor in this case, especially since the tumor arose in the vicinity of the previous piercing site. Trauma-induced chronic inflammation in perforated nipple ducts could create a localized microenvironment that promotes carcinogenesis.
Conflict of interest statement
None declared.
Funding
None declared.



