-
PDF
- Split View
-
Views
-
Cite
Cite
Abraam Rezkalla, Islam Rajab, Nagihan Orhun, Alaa Musallam, Katrina Villegas, Ahmad Nouri, A large colonic polyp: an atypical presentation of follicular lymphoma, Journal of Surgical Case Reports, Volume 2025, Issue 2, February 2025, rjaf058, https://doi.org/10.1093/jscr/rjaf058
Close - Share Icon Share
Abstract
Follicular lymphoma of the gastrointestinal tract is a rare entity accounting for <7% of all non-Hodgkin lymphomas and is more common in the small intestine, whereas colorectal manifestations account for only 1%–2% of the cases. Most patients have limited disease with promising overall survival in comparison to nodal lymphoma although the morphologic, immunophenotypic, and genetic aspects of nodal follicular lymphomas remain the same. Despite the lack of randomized clinical trials, chemotherapy is frequently used to treat NCCN grade III and IV colonic follicular lymphomas with favorable prognosis. We hereby present a case of follicular lymphoma of the colon that was treated conservatively with a successful outcome.
Introduction
Follicular lymphoma (FL) is a subtype of non-Hodgkin lymphoma (NHL) emerging from the germinal focus of B cells [1]. NHL usually arises in the lymph nodes and affects the liver, spleen, and bone marrow [1]. The three most prevalent subtypes of FLs are mucosa-associated lymphoid tissue (MALT) lymphoma, extranodal marginal zone lymphoma, and diffuse large B-cell lymphoma, which account for ~40% of FLs outside the lymph nodes. FL of the gastrointestinal tract is rare and accounts for <7% of all NHLs. The stomach is the first organ to be affected, followed by the small intestine [2, 3]. Colorectal manifestations usually account for merely 1%–2% of the cases [1, 3].
Case report
A 75-year-old female with a past medical history of hypothyroidism underwent her first screening colonoscopy. Colonoscopy showed a flat irregular polyp near the splenic flexure of the colon measuring 20 × 40 mm (Fig. 1). The polyp was resected, and biopsy results were consistent with FL grade I. Immunostaining demonstrated positivity to CD20, BCL-2, CD10, and CD21. Ki-67 staining demonstrated a low proliferation index (10%–20%) in lymphoma cells, with a high proliferation index (>90%) in the residual reactive germinal centers (Fig. 2). The lymphoma cells were negative for CD5 and cyclin 1 (Table 1). Other polyps were found in the proximal transverse colon and rectum, and pathology showed sessile serrated adenomas. Subsequently, the patient underwent PET/CT scan in order to estimate further lymphoma involvement. PET/CT scan revealed no evidence of FL nor increased colonic uptake (Fig. 3).
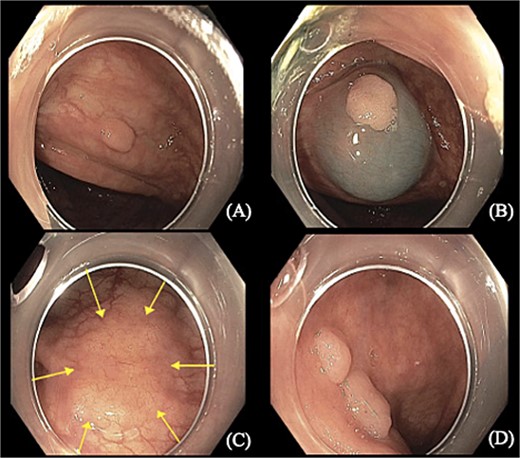
Colonoscopy images show (A, B, and D) sessile polyps found in the proximal transverse colon and rectum. (C) Flat irregular polyp near the colonic splenic flexure measuring 20 × 40 mm.
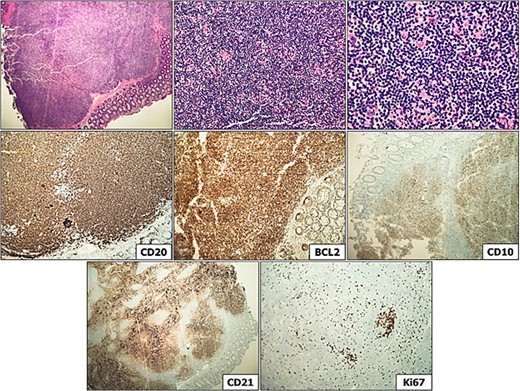
Distal transverse colon polyp, consistent with grade 1 follicular lymphoma. Immunostaining is evidenced above, demonstrating CD20 (lymphocyte positivity), BCL-2 (bright), CD10 (B cell positivity), CD21 (demonstrating markedly expanded and disrupted follicular dendritic meshwork), and Ki-67 staining demonstrating low proliferation index (10%–20%) in lymphoma cells, with high proliferation index (>90%) in the residual reactive germinal centers.
| Antibody . | Result . |
|---|---|
| CD20 | Most of the lymphocytes positive |
| CD3 | Scattered small reactive T-lymphocytes, predominantly at the periphery of B-cell nodules |
| CD5 | B-cells negative |
| CD10 | B-cells positive |
| BCL-2 | Positive |
| BCL-6 | Many cells faintly positive |
| CD43 | Weakly positive to negative |
| Cyclin D1 | Negative |
| CD21 | FDC and many B-cells positive |
| CD35 | FDC positive |
| Ki-67 | 10%–20% in lymphoma cells; >90% in residual reactive germinal centers |
| Antibody . | Result . |
|---|---|
| CD20 | Most of the lymphocytes positive |
| CD3 | Scattered small reactive T-lymphocytes, predominantly at the periphery of B-cell nodules |
| CD5 | B-cells negative |
| CD10 | B-cells positive |
| BCL-2 | Positive |
| BCL-6 | Many cells faintly positive |
| CD43 | Weakly positive to negative |
| Cyclin D1 | Negative |
| CD21 | FDC and many B-cells positive |
| CD35 | FDC positive |
| Ki-67 | 10%–20% in lymphoma cells; >90% in residual reactive germinal centers |
| Antibody . | Result . |
|---|---|
| CD20 | Most of the lymphocytes positive |
| CD3 | Scattered small reactive T-lymphocytes, predominantly at the periphery of B-cell nodules |
| CD5 | B-cells negative |
| CD10 | B-cells positive |
| BCL-2 | Positive |
| BCL-6 | Many cells faintly positive |
| CD43 | Weakly positive to negative |
| Cyclin D1 | Negative |
| CD21 | FDC and many B-cells positive |
| CD35 | FDC positive |
| Ki-67 | 10%–20% in lymphoma cells; >90% in residual reactive germinal centers |
| Antibody . | Result . |
|---|---|
| CD20 | Most of the lymphocytes positive |
| CD3 | Scattered small reactive T-lymphocytes, predominantly at the periphery of B-cell nodules |
| CD5 | B-cells negative |
| CD10 | B-cells positive |
| BCL-2 | Positive |
| BCL-6 | Many cells faintly positive |
| CD43 | Weakly positive to negative |
| Cyclin D1 | Negative |
| CD21 | FDC and many B-cells positive |
| CD35 | FDC positive |
| Ki-67 | 10%–20% in lymphoma cells; >90% in residual reactive germinal centers |
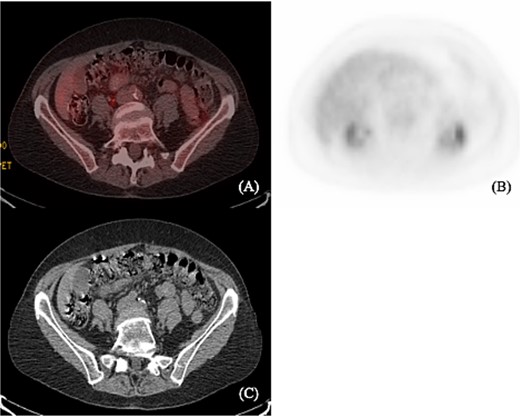
(A) and (B) show axial PET scan sections with 2.2 uptake in the liver. There is no abnormal colonic uptake. Specifically, there is no uptake seen in the splenic flexure corresponding to the mass described on the prior colonoscopy. (C) Computerized tomography shows no mediastinal lymphadenopathy and no axillary lymphadenopathy. Liver and spleen not enlarged. A prominent left inguinal lymph node was noted without evidence of abnormal FDG activity.
Molecular biology studies
B-cell gene rearrangement by PCR was performed and was positive for a clonal B-cell gene rearrangement.
Fluorescence in-situ hybridization (FISH) was performed as well and showed no evidence of BCL2-IGH [translocation t(14;18)] gene rearrangement.
Outcome
PET/CT scan was obtained from the skull to mid-thigh to ensure no further lymphoma involvement. Due to the patient’s resected lesion, no evidence of FL, abnormal colonic uptake, or any uptake in the splenic flexure, which corresponds to the mass on colonoscopy; no further chemotherapy is warranted at this time. The patient underwent a surveillance colonoscopy 6 weeks later, and post-polypectomy scars were biopsied and showed colonic mucosa with mild relative changes and were negative for residual adenomatous changes or lymphoproliferative disorder.
Active surveillance with colonoscopy to be performed in 1 year and PET scan every 6 months was recommended.
Next-generation sequencing cell-free DNA (NGS) was obtained as well on the day of her scan to look for a mutational etiology of this process. Results were negative for PD-L1, ALK Fusion, ROS1 Fusion, NTRK1/NTRK2/NTRK3 Fusion, and MSI or clinically significant variants.
Discussion
Pathophysiology of gastrointestinal FL includes clonal B-cell rearrangement as well as mutations in genes that modify chromatin (including CREBBP and KMT2D), where lesions within tumor cells work to re-educate normal immune cells to aid in cancer proliferation [4]. This disease process involves poorly delineated crowded follicles with centrocytes and displacement of normal structures, similar to nodal lymphoma presentation [4]. Biopsied tissue of those found to have FL, include follicle and monotonous lymphoid cells within the lamina propria, with expression of CD20, BCL-6, BCL-2, and CD-10 along with absence of expression of cyclin D1, CD-5, CD-23, or CD-43 [3, 5–7]. One differentiation noted between extra nodal and nodal lymphoma on biopsy includes follicular dendritic cells (FDCs) throughout the follicles in nodal FL and FDCs arranged in the periphery of neoplastic follicles with extra nodal FL [8]. In our patient’s case, tissue was positive for expression of CD-20, CD-3, CD-10, BCL-2, BCL-6 (faintly positive), CD-21, and CD-35, with negative CD-5 and cyclin D1, consistent with the common histological findings from similar patients with colonic FL.
Per the National Comprehensive Cancer Network (NCCN), primary GI lymphoma can be staged using Lugano criteria [9]. In terms of overall survival (OS), no differences in the OS have been appreciated between treatment with chemotherapy, radiation, combination chemotherapy and radiation, or observation [10]. For patients with a low-tumor burden primary FL presenting without symptoms, there is relatively limited data regarding whether patients benefit from rituximab therapy versus observation [11]. Regardless of whether patients develop the disseminated disease, this particular patient population has a good prognosis [5].
In our case, and given the literature review above, the patient underwent complete resection of the distal transverse polyp with negative extra nodal detection on PET/CT, and would benefit from observation. Our patient has a relatively good prognosis given these findings. She may benefit from additional testing such as LDH as well as hepatitis B testing in the future if she becomes a candidate for therapy. Per NCCN current guidelines, laboratory studies are recommended every 3–6 months for a total of 5 years, followed by annual testing. Surveillance imaging is recommended no more than every 6 months for the first 2 years after diagnosis, followed by annual surveillance afterward.
Manifestations of extra nodal FL are dependent on the location of involvement. Similar to our patient’s case, many patients have been diagnosed with this malignancy as an incidental finding during routine endoscopy [9]. For those who are symptomatic, especially for those patients with extra nodal disease in the small bowel, patients can present with intestinal obstruction [3, 9]. In more serious cases with bulky disease, patients have a risk of intestinal perforation from either the lymphoma or surgical-related complications [12]. In a study investigating gastrointestinal perforations occurring in this specific patient population, perforations most commonly occurred in the small bowel (59%), followed by the stomach (16%), and then the large bowel (22%) [12]. It is important to consider these complications when managing patients with colonic FL. Fortunately, our patient underwent resection of her transverse colon polyp histologically significant for FL with absence of complications.
Acknowledgements
The authors would like to thank the patient and their family for allowing them to share this case with their colleagues. They would also like to thank the Hematology-Oncology and Gastroenterology teams for their assistance in diagnosing and managing the patient’s disease. A special thank you to Dr Sohail Qayyum for his assistance in interpreting the patient’s pathology and contributing biopsy images.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Funding
No funding was received to assist with the preparation of this manuscript.
Ethical approval
Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent to participate
Written informed consent was obtained from the patient himself. The participant has consented to the submission of the case report to the journal.



