-
PDF
- Split View
-
Views
-
Cite
Cite
Ricardo Alonso Beltran Mejía, Lourdes Camacho Ramírez, Jorge Santín Rivero, Alejandro Hoyos Torres, Leopoldo Ernesto Castañeda Martínez, Robotic splenectomy for large splenic cyst with indocyanine green-assisted vascular identification: a case report, Journal of Surgical Case Reports, Volume 2025, Issue 12, December 2025, rjaf992, https://doi.org/10.1093/jscr/rjaf992
Close - Share Icon Share
Abstract
Robotic splenectomy (RS) is an advanced alternative to conventional laparoscopy, offering advantages such as 3D vision and superior articulation, which are crucial in complex cases like splenomegaly. We present the case of a 22-year-old woman with a giant splenic cyst measuring ~13 cm. Robotic-assisted splenectomy was performed using the multi-port Da Vinci Xi system. The robotic approach facilitated the precise dissection of the splenic hilum and the short gastric vessels, allowing the spleen to be extracted intact with minimal bleeding in 2 h. Robotic splenectomy proved to be a safe and effective technique, associated with an efficient operative time and rapid postoperative recovery. This case supports the use of robotic splenectomy in specialized centers for selected, complex cases, demonstrating its technical advantage for large splenic pathology.
Introduction
Splenectomy has undergone a significant evolution in recent decades thanks to the advancement of minimally invasive techniques. Laparoscopy (LS) is the established gold standard for this procedure. However, robotic surgery has emerged as a promising alternative, especially in complex cases where precision and tissue manipulation are crucial. Robotic surgery offers advantages including magnified three-dimensional (3D) vision, greater precision in dissection and tissue manipulation. These benefits are particularly useful in cases of splenomegaly or intra-abdominal adhesions. Previous studies have shown that robotic splenectomy (RS) is associated with less intraoperative blood loss compared to LS [1]. Furthermore, robotic surgery can offer better visualization and precision in cases of splenomegaly [2]. The Surgical CAse REport (SCARE) criteria were followed in reporting this case report [3].
Case presentation
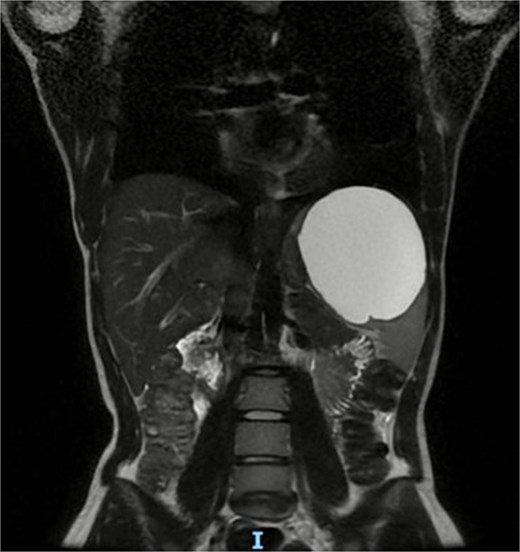
We present a 22-year-old female patient with no relevant past medical history who, 6 months prior to diagnosis, began experiencing mild abdominal pain and distension in the upper abdomen. Laboratory studies showed a white blood cell count of 9500/μl, lymphocytosis of 19%, and neutrophilia of 74.1%. Magnetic resonance imaging reported a giant splenic cyst, ~13 cm (Figs 1 and 2).
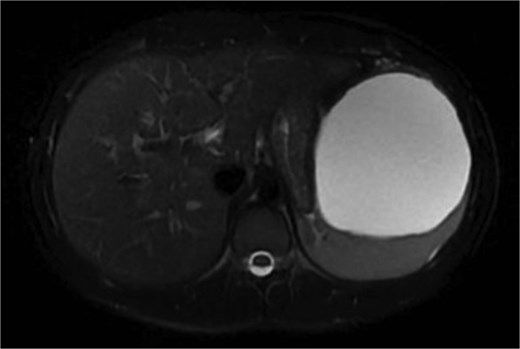
Axial view or preoperative MRI of splenic cyst, diameter of 13 cm.
Splenectomy assisted by the Da Vinci Xi system was performed with the patient in the right lateral decubitus position. The first port was introduced using the open technique at the umbilical level; the remaining ports were placed under direct vision, totaling 5 ports (3 for robotic arms, 2 for camera and assistant).
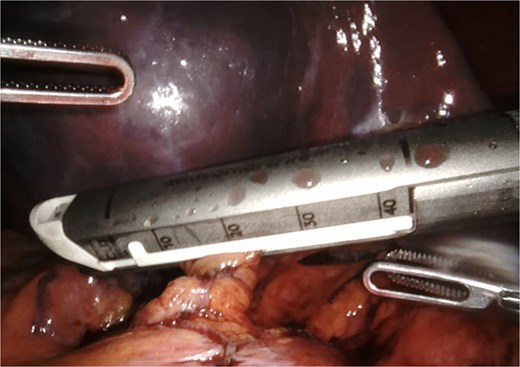
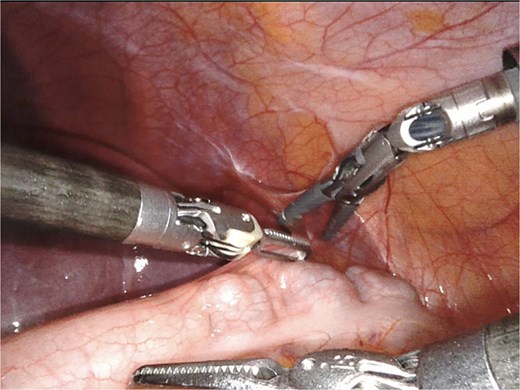
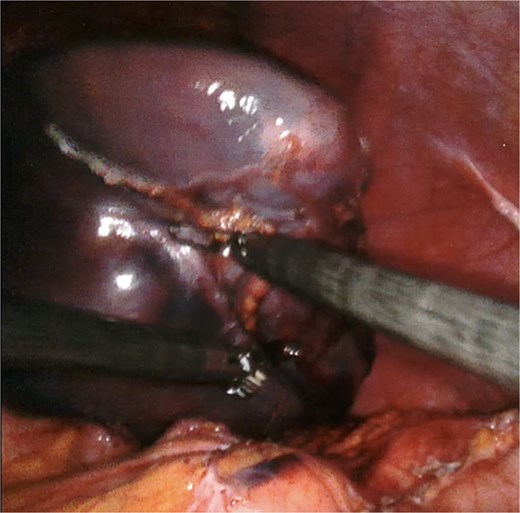
A giant splenic cyst was observed, compressing gastrointestinal organs, with limited mobilization (Fig. 3). Dissection of the splenoepiploic ligaments was performed using a bipolar energy device (Vessel Sealer), followed by dissection of the splenic flexure of the colon. The pre-splenic ligament was divided, and vascular control of the short gastric vessels was achieved. The vascular bed was identified with indocyanine green (ICG)-enhanced fluorescence (Fig. 4), and vascular control of the artery and vein was performed with a 40 mm stapler (Fig. 5). The splenophrenic ligament was divided, and the splenic bed was skeletonized (Fig. 6).
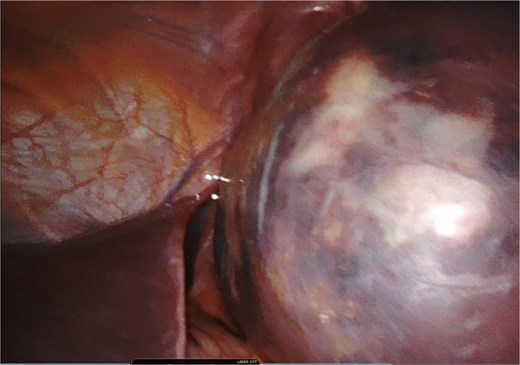
Intraoperative visualization of enlarged spleen and intraparenchymal cyst.
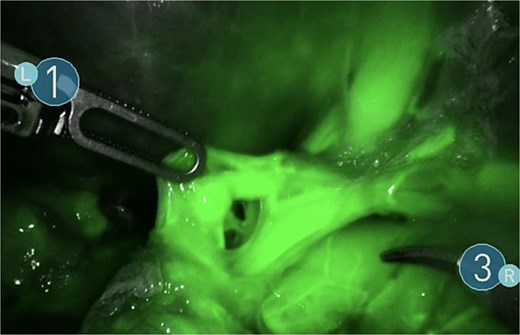
Use of ICG enhanced fluorescence for adequate visualization of splenic hilum.
Once fully mobilized (Fig. 7), the specimen was introduced into a laparoscopic bag to avoid intra-abdominal exposure during extraction (Fig. 8). It was removed intact using an Alexis wound protector through the umbilical port. Minimal blood loss was reported, and the procedure was completed in 2 h. Blake drains were placed. The patient had an adequate postoperative course, discharged home on the second postoperative day, without drains.
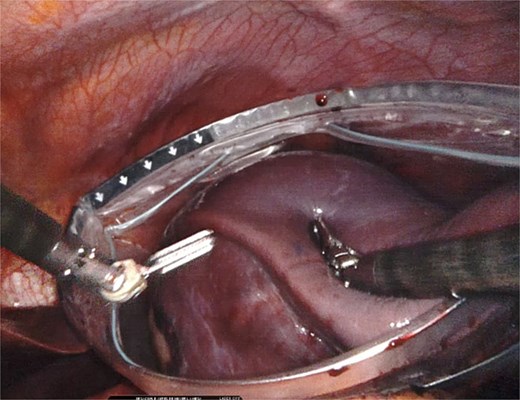
Placement of the spleen in large laparoscopic retrieval bag without rupture of the specimen.
Discussion
RS has established itself as a promising alternative in abdominal surgery, offering advantages compared to conventional laparoscopic techniques. In our case, a 22-year-old patient with a giant splenic cyst underwent a robotic-assisted splenectomy. The results obtained support the benefits described in the literature.
Laparoscopic splenectomy was initially slow to be implemented due to the difficulties presented by the anatomical location, tissue characteristics, blood supply, and cases of splenomegaly that limit the working area. Traditionally, it required longer operative times and carried an increased risk of bleeding. The work carried out by Giza et al. compared 77 robotic splenectomies with conventional LS; they created The Minimally Invasive Splenectomy Score to identify complicated cases, and subsequently categorized them as difficult or simple splenectomies. They found that conventional LS requires a learning curve and longer operative time, concluding that RALS offers advantages in complicated splenectomies [4].
Robotic surgery provided high-definition 3D vision and greater precision in the dissection of the hilar vessels, allowing for safe identification and control of the short vessels and the splenic pedicle, minimizing the risk of bleeding. This advantage aligns with the findings of previous studies that have demonstrated reduced blood loss in RS [1, 5]. Among the most notable advantages, the wider degrees of motion, as well as improved ergonomics for the surgeon, and the use of three articulating arms facilitated the manipulation of the large spleen and dissection. This ability to work in confined spaces is particularly useful in cases of splenomegaly [1].
These features facilitate the manipulation of splenic vessels, leads to a reduced risk of significant bleeding and the need for conversion to open surgery. The decreased bleeding observed in our patient is consistent with previous reports; in a study published by Cavaliere, a significant difference was reported (P = .03) in intraoperative bleeding (100 ml in the RALS group vs. 300 ml in conventional LS) [1]. This may be related to improved articulation allowing better control of the splenic hilum [6].
Conversion rates reported in the literature range from 5.8% to 14% for conventional LS and 0%–2.9% for RALS [1, 2, 5]. A higher conversion rate to open surgery is reported with conventional LS in cases of splenomegaly. Additionally, robotic surgery offers advantages over conventional LS in patients with obesity and previous abdominal surgeries [5, 7].
The patient experienced a rapid postoperative recovery, with an early hospital discharge. This observation is consistent with results reported in previous studies that have demonstrated a shorter hospital stay [5, 8].
There are few studies on the use of adjuncts like ICG-enhanced fluorescence for RS, although its benefits have been largely described in other procedures and can be applied for laparoscopic splenectomy [9].
Studies conducted in the pediatric population have demonstrated the superiority of the robotic technique in operative time, bleeding, and hospital stay [10–12]. In a study published by Shelby et al. reported similar complication rates in both techniques while noting the larger spleen size in the group undergoing RALS [11]. As in the adult population, a higher rate of conversion to open surgery is reported in the conventional LS group [10].
One of the main challenges of adopting robotic surgery is high cost, and in some centers, longer operative times [2, 10–12]. However, even in cases of large spleens, the potential reduction in the need for conversion to open surgery, reduced bleeding, and fewer complications may justify the investment in specialized tertiary centers [13, 14].
Conclusion
RS is a safe and effective for selected patients, offering enhanced precision, improved visualization, and greater dexterity compared with conventional LS. The use of ICG fluorescence can further aid in accurate vascular identification, contributing to safer dissection in cases with distorted anatomy. In our patient with a giant splenic cyst, robotic assistance allowed for intact specimen retrieval with minimal blood loss and an uncomplicated postoperative course. These advantages support the role of robotic surgery as a valuable option in complex splenic pathology.
Conflict of interest statement
None declared.
Funding
None declared.



