-
PDF
- Split View
-
Views
-
Cite
Cite
Zhivorad Kocevski, Andrej Nikolovski, Goran Jović, Viktor Grujevski, Emil Stoicovski, Zan Mitrev, A giant desmoid tumor of the abdominal wall presented as a panniculus morbidus: case report and literature review, Journal of Surgical Case Reports, Volume 2025, Issue 12, December 2025, rjaf1009, https://doi.org/10.1093/jscr/rjaf1009
Close - Share Icon Share
Abstract
The abdominal wall’s desmoid-type fibromatosis (desmoid tumor) is a rare, locally aggressive mesenchymal tumor with no potential for distant metastases. It can occasionally grow to a considerable size, leading to diagnostic confusion and presenting as a challenging surgical problem. This is a case of a giant desmoid tumor of the abdominal wall, which manifested as a panniculus morbidus (apron belly), thus causing an adult-acquired buried penis and reduced quality of life.
Introduction
The desmoid tumor, also called “aggressive fibromatosis” and “desmoid-type fibromatosis” (DF), is a rare mesenchymal malignancy with an incidence of 2–4 cases per million. Its “aggressiveness” is reflected in the high potential for local invasion and recurrence, even though it doesn’t metastasize distantly. Desmoid tumors may arise elsewhere in the body, including the head, neck, extremities, and the abdomen (intra-abdominal or within the abdominal wall) [1]. This is a case of a giant abdominal wall desmoid tumor that was clinically demonstrated as a panniculus morbidus (apron belly) in a male patient. Written informed consent was obtained from the patient for this case report presentation.
Case report
A 54-year-old male patient clinically presented with a massive abdominal pannus, but denied previous weight loss. Body mass index (BMI) was 56.3. The “pannus” has grown to its present size in 2 years. No abdominal trauma or previous surgery was reported. Additionally, an adult-acquired buried penis and separated scrotum containing two testicles with normal anatomy were noted. The patient reported urinating disability, loss of erection, infertility, and was unable to see the penis (Figs. 1 and 2). The preoperative computerized tomography scan showed a massive infra-abdominal mass with predominant fibrous-like tissue, containing a small amount of fat tissue. The patient was admitted for a multidisciplinary approach to surgical treatment.
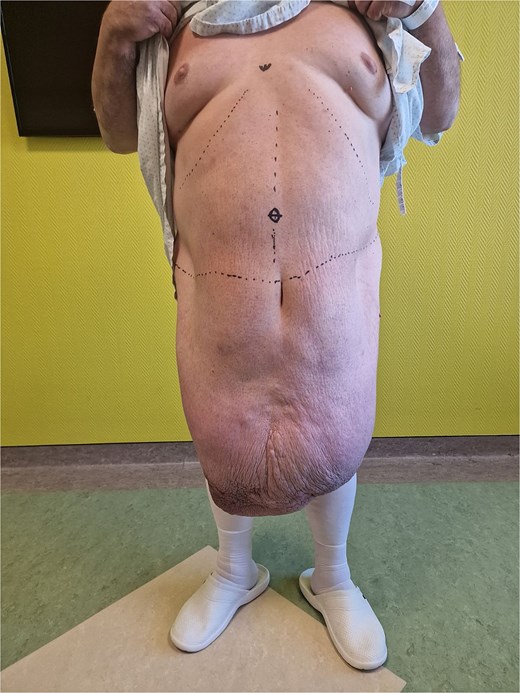
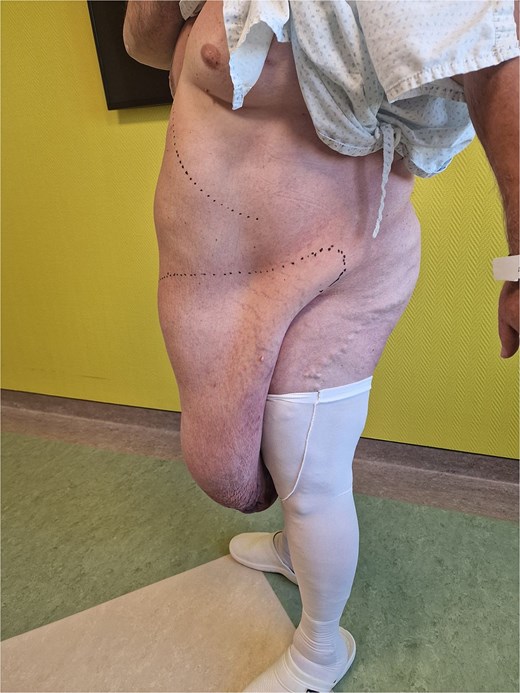
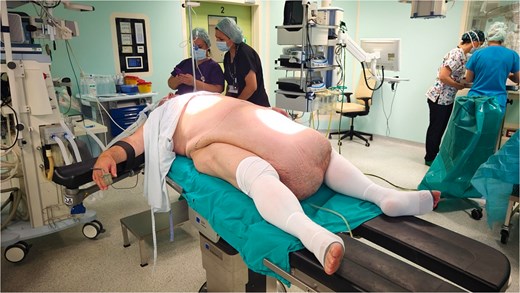
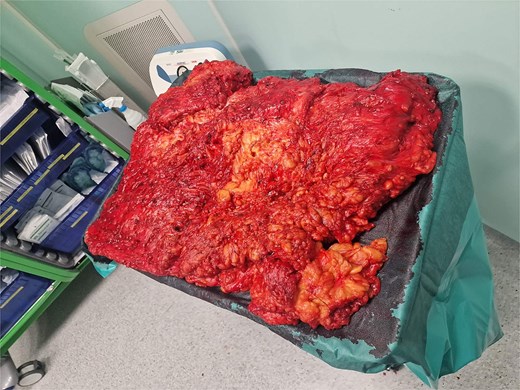
Surgery was performed under general anesthesia. The patient was positioned supine (Fig. 3). A Foley catheter was inserted into the buried penis. Preoperative skin marking was performed as a reference for skin incisions. The lower incision was made along the semicircular marking with a 6 cm radius around the penis. The Foley catheter served as a guide to prevent iatrogenic injury to the penile body. The inferior part of the pannus was mobilized to the muscle fascia. Bilateral lateral incisions were made along the inguinal ligament to the anterior superior iliac spines. The inferior excision extended upward to the umbilical stump. The superior incision was made 2 cm below the previous marking to facilitate planned closure of the abdominoplasty. Subsequently, the superior incision extended down to the muscle fascia and continued in the suprafascial plane to the umbilicus, with its preservation. Following tumor removal, cranial and lateral dissection proceeded in the suprafascial plane up to the xiphoid process and laterally to the rib line. The penile base was released with a partial incision of the suspensory ligament, along with the placement of two sutures anchoring the skin and the fascia of Scarpa on one end to the tunica albuginea of the penis. The preserved umbilicus was transposed to its new position 14 cm proximal to the suture line. Two suction drains were placed beneath the inguinal line. The wound was sutured in layers. Intraoperative blood loss was moderate, and 1 unit of blood was administered after surgery. The operative time was 6.5 h. The removed specimen weighed 35 kg (Fig. 4).
The postoperative period was uneventful. Drains were removed on postoperative Day 3. Postoperative BMI dropped to 43.9. The quality of urination improved to a moderate degree. The length of hospital stay was 6 days. A wound infection with dehiscence occurred and was treated in the outpatient ward with local antibiotics and dressings. Follow-up lasted for 5 months postoperatively (Fig. 5).
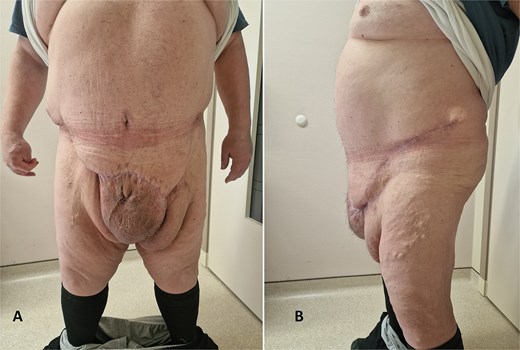
Clinical presentation 5 months after surgery (A—from the front); (B—from the side).
The microscopic pathology report showed thickened stratified squamous epithelium with visible melanin pigment in the basal layer. In the dermal connective tissue, a moderate inflammatory infiltrate consisting of mature lymphocytes and plasma cells was noted. In the deeper dermis and subcutaneous tissue, proliferative, gigantic spindle cells integrated into the collagen stroma were observed. Rare mitoses were noted at a magnification of 20 times. Immunohistochemistry staining indicated positivity for Antigen Kiel 67 (Ki-67) with low proliferative activity of less than 2% and Vimentin (Fig. 6). The final pathology report confirmed the presence of abdominal aggressive fibromatosis (desmoid tumor).
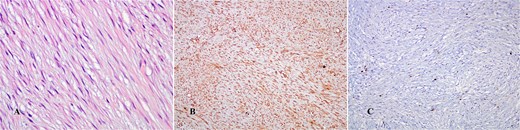
(A) Hematoxylin–eosin stain (×400 HPF); (B) Vimentin positivity (×10 HPF); (C) Ki-67 (×10 HPF).
Discussion
According to the definition from the World Health Organization, desmoid fibromatosis is a clonal fibroblastic proliferation of soft tissues characterized by aggressive infiltrative growth, a tendency to recur, and an absence of metastasis [2]. It is a rare condition, accounting for 0.03% of all neoplasms [3]. The most frequently affected age group is between 30 and 40, with a predominance of females. Two types of this condition are described: sporadic tumors (85%–90%) and cases involving mutations in the adenomatous polyposis coli (APC) gene, which include patients with familial adenomatous polyposis (FAP) syndrome. Risk factors associated with the occurrence of desmoid tumors include trauma, such as prior abdominal surgery, estrogen exposure, and pregnancy, as well as mutations in the APC gene [1].
Giant abdominal wall desmoid tumors are a rare occurrence. To our knowledge, after conducting a literature review, this case presents the heaviest excised specimen containing a desmoid tumor of the abdominal wall ever reported (Table 1).
Known published cases of abdominal wall “giant” desmoid tumor and “desmoid fibromatosis” in English
| Reference . | Author/year . | Patient age/gender . | APC gene mutation status . | Weight of the removed specimen . | Treatment . |
|---|---|---|---|---|---|
| [4] | Gianis TJ et al./1987 | 34/male | 3.76 kg | Laparotomy with partial jejunum resection followed by abdominal wall resection | |
| [5] | Marone U et al./2003 | 37/female | 2 bp deletion at codon 1062 in exon 15, fragment E | 5 kg | Tumor excision followed by abdominal wall reconstruction (Gore-Tex dual mesh prosthesis) |
| [6] | Rakha EA et al./2007 | 38/female | Not reported | 25 kg | Excision of the tumor along with part of the abdominal wall, followed by a plastic procedure |
| [7] | Koshariya M et al./2013 | 25/female | Not reported | 6.5 kg | Tumor excision along with part of the abdominal wall, followed by the release of rectus abdominis, and reconstructed with polypropylene + poliglecaprone 25 (composite mesh) |
| [8] | Kumar P et al./2021 | 38/female | Not reported | Not reported | Excision of the tumor along with part of the abdominal wall, followed by a posterior component separation technique |
| [9] | Patel N et al./2021 | 25/female | Proven | 15 kg | Two-stage surgery: 1. Tumor excision with full-thickness abdominal wall excision + negative pressure 2. Porcine dermal collagen-based matrix mesh sutured to the fascial edges + right anterolateral thigh flap harvested with vastus lateralis muscle and fascia |
| [10] | Zhao J et al./2022 | 24/male | Not reported | Not reported | En bloc resection of the tumor with the underlying musculature, followed by repair with polypropylene mesh |
| [11] | Njoku O et al./2022 | 36/female | Not reported | 6.5 kg | Laparotomy with abdominal wall resection and partial omentectomy followed by primary closure |
| [12] | Dhivakar S et al./2023 | 20/female | Not reported | Not reported | Tamoxifen (no effect) followed by tumor excision along with part of the abdominal wall and bilateral inferiorly based external oblique muscle flap, followed by a mesh repair + adjuvant radiotherapy (60 Gy) |
| [13] | Ghaddou Y et al./2024 | 55/female | Not reported | Not reported | Mono-block resection along with the right rectus abdominis muscle and a part of the anterior aponeurosis of the left rectus abdominis muscle, followed by intraperitoneal synthetic dual mesh placement |
| This case report | Kocevski Z et al./2025 | 54/male | Not examined | 35 kg | Excision followed by abdominoplasty and buried penis repair |
| Reference . | Author/year . | Patient age/gender . | APC gene mutation status . | Weight of the removed specimen . | Treatment . |
|---|---|---|---|---|---|
| [4] | Gianis TJ et al./1987 | 34/male | 3.76 kg | Laparotomy with partial jejunum resection followed by abdominal wall resection | |
| [5] | Marone U et al./2003 | 37/female | 2 bp deletion at codon 1062 in exon 15, fragment E | 5 kg | Tumor excision followed by abdominal wall reconstruction (Gore-Tex dual mesh prosthesis) |
| [6] | Rakha EA et al./2007 | 38/female | Not reported | 25 kg | Excision of the tumor along with part of the abdominal wall, followed by a plastic procedure |
| [7] | Koshariya M et al./2013 | 25/female | Not reported | 6.5 kg | Tumor excision along with part of the abdominal wall, followed by the release of rectus abdominis, and reconstructed with polypropylene + poliglecaprone 25 (composite mesh) |
| [8] | Kumar P et al./2021 | 38/female | Not reported | Not reported | Excision of the tumor along with part of the abdominal wall, followed by a posterior component separation technique |
| [9] | Patel N et al./2021 | 25/female | Proven | 15 kg | Two-stage surgery: 1. Tumor excision with full-thickness abdominal wall excision + negative pressure 2. Porcine dermal collagen-based matrix mesh sutured to the fascial edges + right anterolateral thigh flap harvested with vastus lateralis muscle and fascia |
| [10] | Zhao J et al./2022 | 24/male | Not reported | Not reported | En bloc resection of the tumor with the underlying musculature, followed by repair with polypropylene mesh |
| [11] | Njoku O et al./2022 | 36/female | Not reported | 6.5 kg | Laparotomy with abdominal wall resection and partial omentectomy followed by primary closure |
| [12] | Dhivakar S et al./2023 | 20/female | Not reported | Not reported | Tamoxifen (no effect) followed by tumor excision along with part of the abdominal wall and bilateral inferiorly based external oblique muscle flap, followed by a mesh repair + adjuvant radiotherapy (60 Gy) |
| [13] | Ghaddou Y et al./2024 | 55/female | Not reported | Not reported | Mono-block resection along with the right rectus abdominis muscle and a part of the anterior aponeurosis of the left rectus abdominis muscle, followed by intraperitoneal synthetic dual mesh placement |
| This case report | Kocevski Z et al./2025 | 54/male | Not examined | 35 kg | Excision followed by abdominoplasty and buried penis repair |
Known published cases of abdominal wall “giant” desmoid tumor and “desmoid fibromatosis” in English
| Reference . | Author/year . | Patient age/gender . | APC gene mutation status . | Weight of the removed specimen . | Treatment . |
|---|---|---|---|---|---|
| [4] | Gianis TJ et al./1987 | 34/male | 3.76 kg | Laparotomy with partial jejunum resection followed by abdominal wall resection | |
| [5] | Marone U et al./2003 | 37/female | 2 bp deletion at codon 1062 in exon 15, fragment E | 5 kg | Tumor excision followed by abdominal wall reconstruction (Gore-Tex dual mesh prosthesis) |
| [6] | Rakha EA et al./2007 | 38/female | Not reported | 25 kg | Excision of the tumor along with part of the abdominal wall, followed by a plastic procedure |
| [7] | Koshariya M et al./2013 | 25/female | Not reported | 6.5 kg | Tumor excision along with part of the abdominal wall, followed by the release of rectus abdominis, and reconstructed with polypropylene + poliglecaprone 25 (composite mesh) |
| [8] | Kumar P et al./2021 | 38/female | Not reported | Not reported | Excision of the tumor along with part of the abdominal wall, followed by a posterior component separation technique |
| [9] | Patel N et al./2021 | 25/female | Proven | 15 kg | Two-stage surgery: 1. Tumor excision with full-thickness abdominal wall excision + negative pressure 2. Porcine dermal collagen-based matrix mesh sutured to the fascial edges + right anterolateral thigh flap harvested with vastus lateralis muscle and fascia |
| [10] | Zhao J et al./2022 | 24/male | Not reported | Not reported | En bloc resection of the tumor with the underlying musculature, followed by repair with polypropylene mesh |
| [11] | Njoku O et al./2022 | 36/female | Not reported | 6.5 kg | Laparotomy with abdominal wall resection and partial omentectomy followed by primary closure |
| [12] | Dhivakar S et al./2023 | 20/female | Not reported | Not reported | Tamoxifen (no effect) followed by tumor excision along with part of the abdominal wall and bilateral inferiorly based external oblique muscle flap, followed by a mesh repair + adjuvant radiotherapy (60 Gy) |
| [13] | Ghaddou Y et al./2024 | 55/female | Not reported | Not reported | Mono-block resection along with the right rectus abdominis muscle and a part of the anterior aponeurosis of the left rectus abdominis muscle, followed by intraperitoneal synthetic dual mesh placement |
| This case report | Kocevski Z et al./2025 | 54/male | Not examined | 35 kg | Excision followed by abdominoplasty and buried penis repair |
| Reference . | Author/year . | Patient age/gender . | APC gene mutation status . | Weight of the removed specimen . | Treatment . |
|---|---|---|---|---|---|
| [4] | Gianis TJ et al./1987 | 34/male | 3.76 kg | Laparotomy with partial jejunum resection followed by abdominal wall resection | |
| [5] | Marone U et al./2003 | 37/female | 2 bp deletion at codon 1062 in exon 15, fragment E | 5 kg | Tumor excision followed by abdominal wall reconstruction (Gore-Tex dual mesh prosthesis) |
| [6] | Rakha EA et al./2007 | 38/female | Not reported | 25 kg | Excision of the tumor along with part of the abdominal wall, followed by a plastic procedure |
| [7] | Koshariya M et al./2013 | 25/female | Not reported | 6.5 kg | Tumor excision along with part of the abdominal wall, followed by the release of rectus abdominis, and reconstructed with polypropylene + poliglecaprone 25 (composite mesh) |
| [8] | Kumar P et al./2021 | 38/female | Not reported | Not reported | Excision of the tumor along with part of the abdominal wall, followed by a posterior component separation technique |
| [9] | Patel N et al./2021 | 25/female | Proven | 15 kg | Two-stage surgery: 1. Tumor excision with full-thickness abdominal wall excision + negative pressure 2. Porcine dermal collagen-based matrix mesh sutured to the fascial edges + right anterolateral thigh flap harvested with vastus lateralis muscle and fascia |
| [10] | Zhao J et al./2022 | 24/male | Not reported | Not reported | En bloc resection of the tumor with the underlying musculature, followed by repair with polypropylene mesh |
| [11] | Njoku O et al./2022 | 36/female | Not reported | 6.5 kg | Laparotomy with abdominal wall resection and partial omentectomy followed by primary closure |
| [12] | Dhivakar S et al./2023 | 20/female | Not reported | Not reported | Tamoxifen (no effect) followed by tumor excision along with part of the abdominal wall and bilateral inferiorly based external oblique muscle flap, followed by a mesh repair + adjuvant radiotherapy (60 Gy) |
| [13] | Ghaddou Y et al./2024 | 55/female | Not reported | Not reported | Mono-block resection along with the right rectus abdominis muscle and a part of the anterior aponeurosis of the left rectus abdominis muscle, followed by intraperitoneal synthetic dual mesh placement |
| This case report | Kocevski Z et al./2025 | 54/male | Not examined | 35 kg | Excision followed by abdominoplasty and buried penis repair |
Desmoid tumors present with expansive growth and pain due to the local compression, and most of them are evident even during clinical exams [13, 14]. In this case, the tumor was misinterpreted as a massive pannus and treated as such.
Magnetic resonance imaging is the primary method for diagnosis, local staging, and post-treatment follow-up. However, a biopsy is essential before initiating any kind of treatment. Due to the rarity of this entity, pathology misdiagnosis is reported to be as high as 30%–40% of cases. Therefore, consulting an expert in soft-tissue pathology is advisable for an opinion [2]. In this case, preoperative biopsy was not undertaken.
The update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma Patients EuroNet and the European Organization for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group states that surgery is no longer a standard treatment, particularly for asymptomatic patients, as a 50% progression-free survival rate is reported with a conservative “watchful waiting” strategy. Another advantage of non-operative treatment is the spontaneous regression rate of 20%–30% in cases, regardless of the tumor site. Other treatment strategies include isolated limb perfusion (with tumor necrosis factor alpha and melphalan), cryoablation (for progressive, locally advanced DF of the limbs), radiotherapy (intensity-modulated radiotherapy and image-guided radiotherapy), combined radiotherapy and surgery (for recurrent DF and cases with incomplete surgical resection), and medical (systemic) therapy using non-steroidal anti-inflammatory drugs, tamoxifen, low-dose chemotherapy, tyrosine kinase inhibitors, and full-dose chemotherapy [2]. Desmoid tumors of considerable size are reported to be treated with upfront surgery (Table 1), which makes sense considering that these tumors often present with specific symptoms. In this case, surgery was chosen due to diagnostic uncertainty and the presence of several symptoms, combined with the patient’s reduced quality of life.
Recurrence is a well-known problem in abdominal wall DF. Factors associated with recurrence include female gender, location, size greater than 7 cm, and younger age. Treatment strategies for abdominal wall DF recurrence should consider minimizing tissue loss and avoiding creating large defects, as these can complicate abdominal wall closure [3].
Prognosis is dependent on the amount of local destruction and has a high morbidity. However, mortality is the reality only in patients with FAP syndrome [15].
Conclusion
This case illustrates the successful removal of a desmoid tumor manifesting as panniculus morbidus in a male patient with a severely impaired quality of life. The excised specimen is among the heaviest ever reported.
Conflict of interest statement
None declared.
Funding
None declared.
References
Dhivakar S, Huda F, Singh SK, et al. Giant anterior abdominal wall desmoid tumor successfully managed with abdominal wall reconstruction.



