-
PDF
- Split View
-
Views
-
Cite
Cite
Anupam K Gupta, Harsha Polavarapu, Kirk Hall, Metastatic sacral chordoma presenting as a cecal polyp: a case report, Journal of Surgical Case Reports, Volume 2025, Issue 12, December 2025, rjaf1005, https://doi.org/10.1093/jscr/rjaf1005
Close - Share Icon Share
Abstract
Chordoma is a rare malignant tumor arising from notochordal remnants, typically occurring along the axial skeleton between the skull base and sacrum. Although chordomas are locally aggressive, they can metastasize—most commonly to the lungs, followed by bone, lymph nodes, and liver. We report an unusual case of metastatic chordoma presenting as a pedunculated cecal polyp. A 67-year-old male with a history of sacral chordoma and prior metastases to lung and bone, under treatment with proton beam radiation, presented with intermittent lower gastrointestinal bleeding. Colonoscopy revealed a 1-cm pedunculated polyp in the cecum, which was completely resected by snare polypectomy. Histopathology demonstrated chondroid material with atypical cell clusters within colonic mucosa. Immunohistochemistry was strongly positive for pancytokeratin and brachyury, confirming the diagnosis of metastatic chordoma. While pulmonary metastasis is the most common presentation of disseminated chordoma, this case highlights an unusual site of metastatic spread to a colonic polyp, underscoring the importance of thorough histopathological and immunohistochemical evaluation of polyps in patients with a history of chordoma.
Introduction
Chordoma is a rare, slow-growing malignant neoplasm that arises from remnants of the embryonic notochord [1]. These tumors account for approximately 1%–4% of all primary bone malignancies and may occur anywhere along the craniospinal axis, most commonly in the clivus and sacrococcygeal region [2]. Chordoma typically affects individuals between 40 and 60 years of age with a slight male predominance in sacral lesions [3].
Histologically, chordomas are characterized by lobules of physaliphorous cells within a myxoid matrix. Immunohistochemistry demonstrates positivity for cytokeratins, S-100, epithelial membrane antigen, and nuclear expression of brachyury, a transcription factor that serves as a highly sensitive and specific marker [4].
Chordoma has a high rate of local recurrence despite surgical resection and proton-based radiotherapy [5]. Metastatic spread occurs in up to 40% of cases, with the lungs being the most common site, followed by bone, lymph nodes, and liver [6]. Metastatic involvement of the gastrointestinal tract is extremely rare. Here, we present a case of metastatic sacral chordoma discovered as a cecal polyp during evaluation for lower gastrointestinal bleeding.
Case presentation
A 67-year-old male presented for evaluation of intermittent lower gastrointestinal bleeding. His medical history was significant for a sacral chordoma with metastases to the lungs and bones, currently managed with proton beam radiation therapy. Comorbidities included diverticular disease, depression, hypothyroidism, and chronic pain requiring narcotic analgesics.
Laboratory investigations, including complete blood count and metabolic panel, were within normal limits. Physical examination, including perineal and digital rectal examination, was unremarkable. Colonoscopy revealed a 1-cm pedunculated polyp in the cecum, with gross appearance suggestive of a tubular adenoma. Snare polypectomy was performed with complete retrieval of the specimen.
Histopathological analysis revealed chondroid matrix with nests of atypical epithelioid cells infiltrating the colonic mucosa. Immunohistochemistry showed strong nuclear positivity for brachyury and diffuse pancytokeratin staining, consistent with metastatic chordoma (Figs 1– 3).
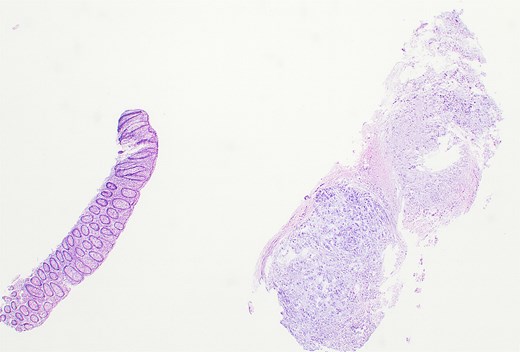
Hematoxylin and eosin–stained section showing colonic mucosa with nests of atypical epithelioid cells within a chondroid matrix, consistent with metastatic chordoma (×10).
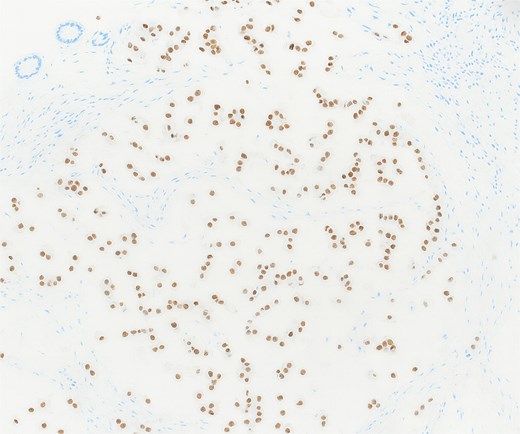
Immunohistochemistry showing strong nuclear positivity for brachyury, confirming the diagnosis of metastatic chordoma (×10).
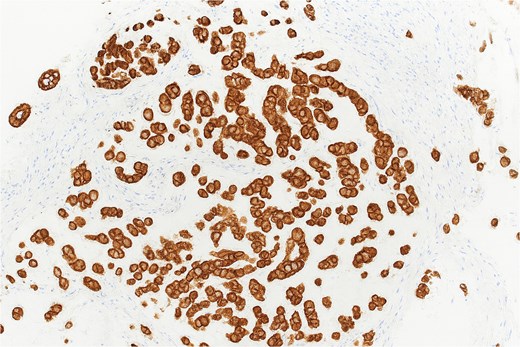
Pancytokeratin immunohistochemistry demonstrating diffuse membranous and cytoplasmic positivity in tumor cells (×40).
Discussion
Chordoma is a malignant neoplasm arising from notochordal remnants, most commonly located in the sacrococcygeal region (50%) and clivus (35%) [2, 7]. Although slow growing, chordomas are locally destructive and carry a significant risk of recurrence even after en bloc surgical resection [6, 7].
Metastatic spread is more frequent in larger tumors, high-grade or dedifferentiated chordomas, and in recurrent disease. The lungs are the predominant site of metastasis, accounting for more than half of cases, followed by bone, lymph nodes, and liver [8]. Metastasis to the gastrointestinal tract is exceedingly rare, with very few cases reported in the literature [8–10].
Histopathologically, chordomas exhibit lobulated growth with physaliphorous cells in a myxoid stroma. Immunohistochemical staining is crucial for diagnosis, with brachyury serving as a highly specific marker [11]. Management of metastatic chordoma is challenging and primarily involves surgery and high-dose conformal radiotherapy (e.g. proton or carbon ion therapy). Systemic therapies are largely palliative, as no definitive chemotherapy regimen has been established [12].
Our case highlights the importance of considering metastatic disease in the differential diagnosis of colonic polyps in patients with a history of chordoma [8, 9, 13]. The presence of brachyury positivity was key to confirming the metastatic nature of this lesion [14].
Conclusion
Metastatic chordoma most commonly involves the lungs but may rarely present as colonic polyps. This case underscores the need for histopathological and immunohistochemical analysis of gastrointestinal lesions in patients with a prior history of chordoma, as early detection of metastases can guide therapeutic decision-making and surveillance strategies.
Author contributions
A.K.G.: case conception, drafting, patient care; H.P.: surgical management, literature review; K.H.: pathological examination, interpretation and revision. All authors approved the final manuscript and are accountable for all aspects of the work.
Conflict of interest statement
None declared.
Funding
None declared.
Patient consent
Written informed consent for publication of clinical details and images was obtained.



