-
PDF
- Split View
-
Views
-
Cite
Cite
Joelle Milan, Carine El Hajj, Ahmad Jradi, Anthony Azar, Karam Karam, Clemence Matta, Bachir Elias, Huge mucinous ovarian borderline tumor: a case report, Journal of Surgical Case Reports, Volume 2025, Issue 12, December 2025, rjaf1000, https://doi.org/10.1093/jscr/rjaf1000
Close - Share Icon Share
Abstract
We present a case report of an atypical 9 kg intestinal like borderline mucinous ovarian cancer in a 58-year-old postmenopausal woman presenting with ascites, increased abdominal circumference, weight loss, and asthenia. A computed tomography scan revealed a 240 × 180 × 235 mm multilobulated abdominal mass associated with left pleural effusion and atelectasis. A 9 kg mass was resected by laparotomy with combined hysterectomy salpingo-oophoprectomy and appendectomy and multiple biopsies were taken from the omentum, surrounding tissues, and peritoneal walls. The patient was discharged with a wait and see follow up plan. Borderline ovarian tumors are a rare entity of malignancies, they usually occur in younger patients, are asymptomatic without high levels of tumor markers (CA19.9 > 10 000). Extensive surgical resection is still debated as most of these tumors occur in child-bearing age women. With the increasing number of atypical presentations, there is a greater need to generate evidence-based guidelines to guide the management of this disease.
Introduction
Nowadays, massive ovarian tumors are rare in advanced countries due to regular health checks and advanced screening. Mucinous borderline ovarian tumors present usually with a bulky multi-lobulated cystic mass with an average size of 18 cm [1]. It affects women between 20 and 40 and manifests with abdominal compressive symptoms [1].
This is a case report of a unique 9 kg mucinous borderline mass that evolved stealthily over several years. Various cases of mucinous ovarian tumors have been reported however the tumor size was inferior along with an early presentation [2, 3]. Our case report represents the natural evolution of this tumor. The diagnosis, treatment, and prognosis of this disease are still highly debatable with scarce publications. Therefore, every contribution to the literature is valuable to generate an evidence-based treatment.
Case presentation
A 58-years-old female previously healthy, presented with gradual increase in the abdominal circumference and a massive weight loss of 17 kg over the past 6 months (Body Mass Index, BMI = 18). She had her menarche at 13 and her menopause at 41. The patient conceived one child without fertility or hormone therapy. Computed tomography (CT) showed an enormous abdominal tumor with minor peritoneal effusion, atelectasis, and pleurisy over the right lung (Figs 1 and 2).
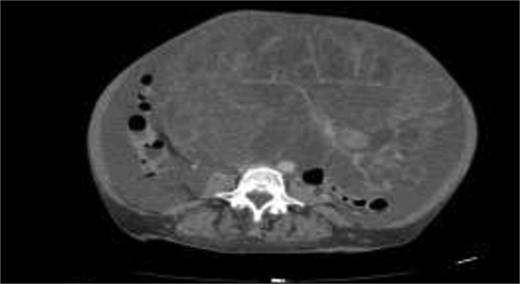
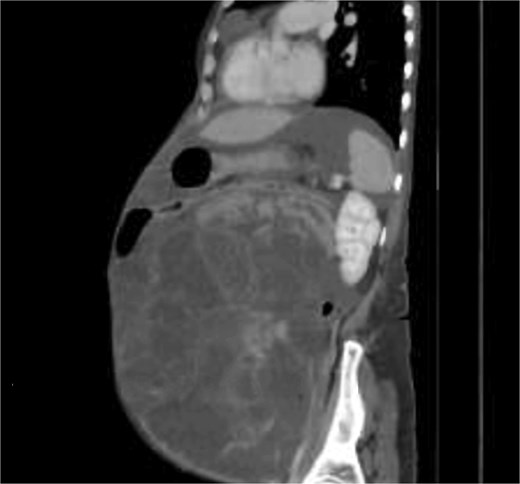
Pleural and peritoneal cultures had negative anatomopathological results and showed exudate with lymphocytic predominance. Tuberculosis was ruled out (QuantiFERON test negative). Tumor markers levels shown in Table 1.
Pre and post operative tumor markers levels, reference ranges from Yang et al. [4].
| Tumor Marker . | Reference Range . | Pre-operative Value . | Post-operative Value . |
|---|---|---|---|
| CA 19.9 | <37 U/mL | > 10 000 | 55.4 |
| CA 125 | 0–35 U/mL | ≈ 350 | 59.7 |
| CEA | < 5 ng/mL (non-smokers) | Not available | 1.54 |
| < 3 ng/mL (smokers) |
| Tumor Marker . | Reference Range . | Pre-operative Value . | Post-operative Value . |
|---|---|---|---|
| CA 19.9 | <37 U/mL | > 10 000 | 55.4 |
| CA 125 | 0–35 U/mL | ≈ 350 | 59.7 |
| CEA | < 5 ng/mL (non-smokers) | Not available | 1.54 |
| < 3 ng/mL (smokers) |
Pre and post operative tumor markers levels, reference ranges from Yang et al. [4].
| Tumor Marker . | Reference Range . | Pre-operative Value . | Post-operative Value . |
|---|---|---|---|
| CA 19.9 | <37 U/mL | > 10 000 | 55.4 |
| CA 125 | 0–35 U/mL | ≈ 350 | 59.7 |
| CEA | < 5 ng/mL (non-smokers) | Not available | 1.54 |
| < 3 ng/mL (smokers) |
| Tumor Marker . | Reference Range . | Pre-operative Value . | Post-operative Value . |
|---|---|---|---|
| CA 19.9 | <37 U/mL | > 10 000 | 55.4 |
| CA 125 | 0–35 U/mL | ≈ 350 | 59.7 |
| CEA | < 5 ng/mL (non-smokers) | Not available | 1.54 |
| < 3 ng/mL (smokers) |
The patient was scheduled for a diagnostic laparoscopy which revealed 3 L of ascites, no peritoneal carcinomatosis and a large polylobate partially cystic tumor arising from the pelvis. The mass was resected in one piece through xypho-pubic laparotomy. It weighted 9 kg (240 × 180 × 235 mm) and had relatively thick walls attached to the left ovary (Figs 3–5). A concomitant total hysterectomy, right adnexectomy, omentectomy, and appendectomy were performed with multiple biopsies from the Douglas, liver, omentum, mesenteric, peritoneal, and pelvic walls.
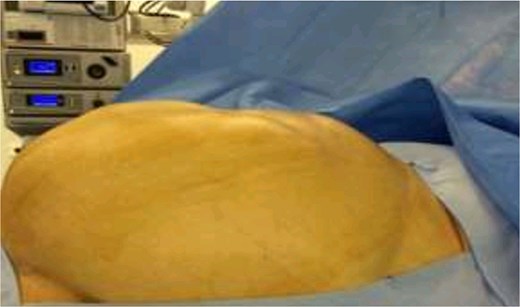
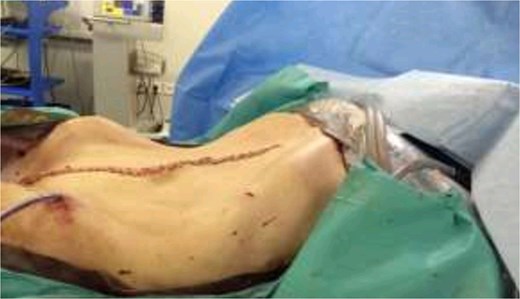
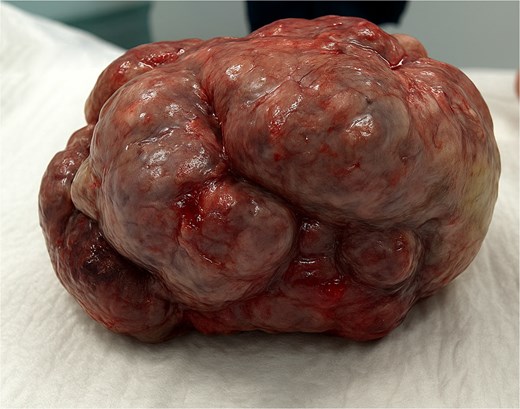
Patient recovered in the intensive care unit with a chest drain then was transferred to the general surgery floor and discharged in good condition 1 week post-operatively. Anatomopathological examination revealed an intestinal-type mucinous partially papillary borderline ovarian tumor with no signs of adenocarcinomatosis. The other biopsies had normal histopathological results. One-month post-surgery tumor markers levels are shown in Table 1. The patient received no chemotherapy and we adopted a wait and see approach.
Discussion
So far, chemotherapy is not advised for borderline ovarian tumors unless diagnosed with advanced disease and even in advanced stages, chemotherapy is controversial [2]. This is why we adopted a wait-and-see approach. Survival is based on the stage of diagnosis and the histological characteristic of the tumor [3]. Risk of recurrence after a conservative approach is ˂30% with a borderline histology and a 13% cumulative risk of recurrence as invasive carcinomas [3, 5]. Lymphadenectomy is not recommended as studies have showed high survival at 6 years for women with lymphatic involvement [6]. Conservative vs invasive surgery is still debatable but unilateral salpingo-oophorectomy is an option in case of a unilateral disease [6].
Borderline tumors are characterized by the presence of an epithelial proliferation without a stromal invasion [5, 6]. Classically they affect young women (below 40), are diagnosed in early stages and have a good prognosis [1, 5, 7].
Mucinous borderline carcinomas are divided into: endocervical like (EMBT) and intestinal like (IMBT) [5]. IMBT have goblet endocrine absorptive cells and rare Paneth cells, whereas the EMBT have polygonal eosinophilic cells with no intestinal differentiation [5]. Clinically, IMBT are usually unilateral with a larger mean size than EMBT, and fewer association with endometriosis [1, 5]. IMBT have a 90% 7-year recurrence free survival and occur at an older age compared to the EMBT [5]. Mucinous tumors of the ovaries can be secondary to cancerous metastasis from the large intestine, kidneys, breasts, appendix, and gallbladder [5]. Some mucinous masses invading the ovaries are concomitant with pseudomyxoma peritonei and therefore originate from the appendix [5]. Additionally, borderline and mucinous ovarian tumor can be the origin of a pseudomyxoma peritonei hence the need for an appendectomy [1, 5, 8]. Furthermore, the atypical presentation of our case which is more consistent with a typical advanced pseudomyxoma peritonei presentation supported the need for an appendectomy to better assess the origin of this tumor and improve the prognosis.
Immunohistochemistry helps determine the tumor origin since metastatic tumors from the intestines have CK7-/CK20+ results unlike primitive ovarian tumors [5]. Thus far, there is still no certain evidence of an association between the BRCA mutation and this type of tumors [7].
CA125 is not considered a useful diagnostic tool as patients usually don’t have values that exceed 100 units/ml [1]. High values could hint toward an ovarian cancer. Our patient had a mucinous borderline disease despite high levels of CA125 and CA19.9. This highlights the importance of the histopathological testing as the standard tool of diagnosis and staging of mucinous borderline tumors [1, 8]. Perioperative histological studies on multiple biopsies from the peritoneum, the mass, and the surrounding tissues (fallopian tubes, diaphragm omentum) should be a standard diagnostic tool.
MRI helps differentiate borderline from malignant mucinous ovarian tumors based on size, shape, margins, presence of papillae, abundance of cysts and wall septation, presence of solid components [9]. Mucinous borderline tumors have less prominent papillae and solid components and low enhancement ratio with fewer instances of moderate ascites [9]. A retrospective study done in Nottingham showed that adequate debulking surgery in both borderline and malignant mucinous ovarian tumors have better prognosis and a higher 5-year survival rate (>70% debulked) however, surgery did not reduce the risk of recurrence of both types [10].
Conclusion
Mucinous borderline tumors are a rare form of ovarian tumors with highly heterogenous presentations. Lack of appropriate management can severely impact surrounding tissues and worsen the prognosis. It is crucial to highlight the importance of implementing new diagnostic and screening techniques among routine gynecological checkups to assess for this type of tumor. The signs of an increased incidence over the years, and the lack of evidence-based guidelines are an incentive to publish case reports discussing the presentation and management of this disease.
Conflict of interest statement
None declared.
Funding
The authors received no financial support for the research and authorship of this article.
Ethical considerations
Our institution does not require ethical approval for reporting individual case reports.
Informed consent
Signed written informed consent for writing and publication was obtained.
References
- ascites
- abdominal mass
- computed tomography
- pleural effusion
- biopsy
- weight reduction
- atelectasis
- appendectomy
- cancer
- debulking
- asthenia
- child
- follow-up
- hysterectomy
- intestines
- laparotomy
- omentum
- ovarian neoplasms
- postmenopause
- pseudomyxoma peritonei
- surgical procedures, operative
- guidelines
- neoplasms
- peritoneum
- surgery specialty
- ovarian cancer
- tumor marker
- salpingo-oophorectomy
- evidence-based practice
- ovarian tumor, borderline
- excision
- abdominal circumference
- borderline neoplasms



