-
PDF
- Split View
-
Views
-
Cite
Cite
Shinichi Ishida, Tsutomu Ihara, Akihiro Mori, Kei Yagami, Retrograde type A dissection and rupture of the aortic root during endovascular aortic repair, Journal of Surgical Case Reports, Volume 2025, Issue 10, October 2025, rjaf823, https://doi.org/10.1093/jscr/rjaf823
Close - Share Icon Share
Abstract
Retrograde type A dissection (RTAD) is a rare but catastrophic complication of thoracic endovascular repair. However, only a few cases of RTAD associated with endovascular aortic repair (EVAR) have been reported. Additionally, aortic root rupture has not been reported. An 80-year-old woman presented with a progressively enlarging intrarenal abdominal aortic aneurysm, and she underwent EVAR. However, cardiac tamponade occurred because RTAD occurred at the end of the surgery, and the aortic root ruptured. Therefore, ascending aortic replacement was performed emergently, and the patient was saved. RTAD with EVAR is rare, but it could be a crucial complication. We could rescue the patient by early detection and surgery. Even with EVAR, the possibility of performing an open chest should be considered. Moreover, fluoroscopy could be used immediately if cardiac tamponade is suspected.
Introduction
Retrograde type A dissection (RTAD) is a rare but catastrophic complication of thoracic endovascular repair [1]. The management of RTAD is currently not standardized, with reported successful outcomes following medical therapy, open surgery, or endovascular repair [2]. Depending on the patient’s clinical condition, emergent decisions and treatments may be required. However, only a few cases have been reported on RTAD with endovascular aortic repair (EVAR). Additionally, cases of aortic root rupture have not been reported. Here, we present a case of RTAD and aortic root rupture during EVAR for an abdominal aortic aneurysm (AAA).
Case
An 80-year-old woman presented with a progressively enlarging intrarenal AAA, measuring up to 50 mm. The patient’s performance status was relatively low; therefore, EVAR was selected to treat the AAA instead of open surgery. EVAR was performed under local anesthesia, and the procedure was conducted as usual (Gore Excluder 23 mm × 14.5 mm × 12 cm; W. L. Gore & Associates, Inc.; Delaware, USA). Ballooning was performed after implanting the main body stent graft and iliac limb extension stent graft. Subsequently, aortography was performed, which showed a type 1a endoleak. Therefore, ballooning was added at the top of the main body stent graft; however, the endoleak persisted. Thus, an additional stent graft (Gore Excluder 23 mm × 4.5 cm) was implanted at the upper end of the main body stent graft. Aortography was performed after ballooning; however, the flow was stagnating in the stent graft. At the same time, the pulse rate of the patient decreased suddenly, and she lost consciousness. She had pulseless electrical activity. Cardiopulmonary resuscitation was initiated, and an aortography was performed again. The presence of an aortic dissection was unclear; however, massive pericardial effusion was confirmed (Fig. 1). Cardiac tamponade by RTAD was suspected, and median sternotomy was performed emergently. After pericardiotomy, the pericardial cavity was filled with blood and hematoma, and spontaneous pulse returned after their removal. However, the adventitia of the aortic root was ruptured, and bleeding persisted. Therefore, ascending aortic replacement or aortic root replacement was performed. Cardiopulmonary bypass was established via left femoral artery perfusion and bicaval drainage. After the nasopharyngeal temperature reached 22°C, circulatory arrest was initiated. The ascending aorta was incised, and the lumen was checked. No tear was found at the intima of the ascending aorta and aortic root. Therefore, ascending aortic replacement was performed (Triplex Advanced 26 mm, Vascutek Terumo, Tokyo, Japan) with retrograde cerebral perfusion. The operation was completed without incident. The patient was extubated on the first postoperative day, and the postoperative course was uneventful. The postoperative enhanced computed tomography (CT) revealed that the entry of the dissection existed at the top of the stent at the abdominal aorta, and the dissection was connected to the distal side of the ascending aortic artificial graft (Fig. 2). The patient was discharged 30 days postoperatively. At the 1-year follow-up, the aorta treated for dissection remained stable.
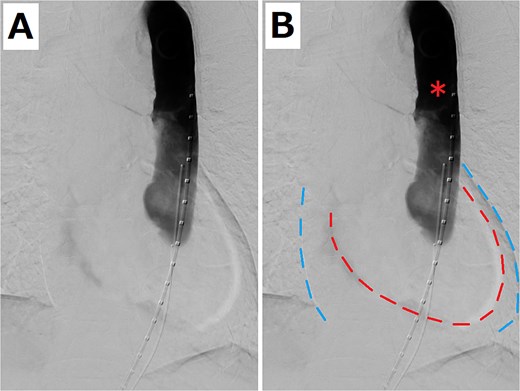
(A) Aortography when the patient had pulseless electrical activity. (B) Pericardial effusion is shown. The red and blue dashed lines indicate the edge of the heart and pericardium, respectively. The red asterisk shows that the flow was stagnating in the stent graft.
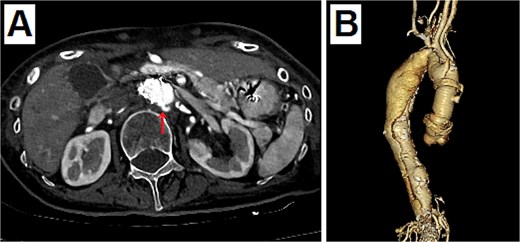
Postoperative enhanced CT. (A) The entry of dissection existed at the top of the stent at the abdominal aorta (arrow). (B) The 3D CT viewed from the right side. The dissection was connected to the distal side of the ascending aortic artificial graft from the top of the stent at the abdominal aorta.
Discussion
RTAD after thoracic endovascular repair has been reported approximately 0.9%–6.8% [3]. It often requires ascending aorta, aortic root, or arch reconstruction depending on cases with hypothermic circulatory arrest. The mortality rate was reported to be 27%–57% [4]. However, the incidence of RTAD intra- or after EVAR has not been reported. In this case, the dissection occurred intraoperatively during EVAR for AAA, reaching the aortic root and rupturing the adventitia. The cause of the severe condition may be due to two points. First, it occurred at the end of the surgery. The aortic dissection occurred from the top of the abdominal aortic stent, and the pressure in the dissected lumen could not escape to the distal side because of the completed abdominal stent graft. Thus, the proximal side of the aorta may have suffered from severe pressure, causing a rupture of the adventitia. Second, the patient was heparinized and not injected with protamine because the EVAR was still ongoing. This may have contributed to the dissection. Other factors may also have contributed to this occurrence, such as the elderly case and low performance status.
To prevent RTAD with EVAR, excessive stent addition and ballooning should be avoided, particularly in elderly and frail patients. However, complete prevention is difficult as endoleaks should be avoided. Therefore, early detection and handling are important. Even with EVAR, the possibility of dissection should be considered, and being prepared to open the chest immediately is crucial. Additionally, fluoroscopy could show pericardial effusions clearly. Thus, fluoroscopy should be used frequently to check for cardiac tamponade when suspected.
Conclusion
RTAD with EVAR is very rare but a crucial complication. We could rescue the patient by early detection and surgery. We should consider that opening the chest may be necessary, even with EVAR. Moreover, fluoroscopy can be used immediately if cardiac tamponade is suspected.
Conflict of interest statement
None declared.
Funding
None declared.



