-
PDF
- Split View
-
Views
-
Cite
Cite
Yue Dong, Liman Zhang, Qiang Wang, Lili Wang, Tianpeng Zhang, Fei Ju, Jie Yang, Polidocanol foam injection sclerotherapy in patients with mixed hemorrhoids with severe anemia: a case report, Journal of Surgical Case Reports, Volume 2025, Issue 10, October 2025, rjaf801, https://doi.org/10.1093/jscr/rjaf801
Close - Share Icon Share
Abstract
Hemorrhoids, a prevalent proctological disease, often present with bleeding, which can lead to severe anemia. Anemia exacerbates anesthesia and surgical risks, raises postoperative infection and mortality rates, and slows recovery. Sclerotherapy for anemia-related hemorrhoids is considered relatively safe and effective. Here, we report a case of sclerotherapy for severe anemia-related hemorrhoids, aiming to provide a reference for the diagnosis and treatment of comparable patients.
Introduction
Hemorrhoids are a common proctologic condition, and bleeding represents one of their cardinal manifestations. Epidemiologic data indicate that ~1 in every 200 000 patients with hemorrhoids may develop anemia [1].The presence of anemia can increase the risks associated with anesthesia and surgery [2] and is also an independent influencing factor for postoperative infection [3]. Polidocanol sclerotherapy is a well-established technique for the treatment of hemorrhoids. It induces local endothelial damage, leading to thrombus formation and fibrosis, thereby reducing the size of the hemorrhoidal mass and achieving hemostasis [3]. This case report details the successful application of polidocanol sclerotherapy in a patient with mixed hemorrhoids and severe anemia, highlighting its efficacy and safety in a complex clinical scenario.
Case report
A 41-year-old male was admitted to the hospital with the chief complaint of “recurrent hematochezia for over ten years, exacerbated in the past six months.” On specialized examination, rectoscopy revealed circumscribed mucosal hyperemia, protrusion, and punctate bleeding at the 3, 7, and 11 o’clock positions above the dentate line in the lithotomy position. Auxiliary examinations showed the following: (1) red blood cell count 2.07 × 1012/L and hemoglobin 52 g/L on complete blood count; (2) liver function, renal function, coagulation function, and indicators related to infectious diseases all within normal limits; and (3) sinus tachycardia (115 beats per minute) on electrocardiogram. The patient was preliminarily diagnosed with mixed hemorrhoids and severe anemia. Given that the persistent bleeding could further exacerbate the anemia, and after excluding contraindications for treatment, polidocanol foam sclerotherapy was performed under local infiltration anesthesia.
The polidocanol foam sclerosant was prepared using the Tessari method [4]: one 10-ml syringe was filled with 8 ml of air, and another 10-ml syringe was filled with 2 ml of polidocanol. The two syringes were connected via a three-way device (Fig. 1A). By rapidly and repeatedly pushing and pulling the plunger of the syringes, the air and sclerosant were thoroughly mixed to create a uniform, white, fine-foam polidocanol sclerosant (Fig. 1B). The patient was placed in the lithotomy position, and the surgical field was disinfected routinely. Local infiltration anesthesia with 1% lidocaine was administered around the anus. A proctoscope was inserted to identify the location, distribution, and size of the hemorrhoids. Immediately after the preparation of the polidocanol foam sclerosant, the injection was initiated. A 0.5-mm-diameter needle was used to minimize the risk of bleeding at the puncture site (Fig. 1C). The injection sites were located at the base of the hemorrhoids above the dentate line (Fig. 2A), with the depth controlled to the submucosal layer. Multiple injection sites were selected (at the 3, 7, and 11 o’clock positions, targeting the dilated hemorrhoidal venous plexus), with 0.4–2 ml of sclerosant foam injected per site, for a total volume of 2–10 ml. The injection was continued until the hemorrhoid surface became visibly pale and elevated (Fig. 2B and C). After the injection, the area was gently massaged to facilitate drug absorption. Vaseline gauze was packed into the anus to apply pressure and prevent bleeding, and sterile dressing was applied to secure the packing in place.
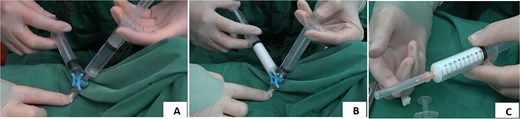
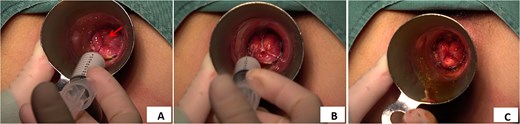
Injection method of polidocanol foam sclerosing agent. (A) Injection site of polidocanol foam sclerosant (indicated by arrow). (B) Injection treatment with polidocanol foam sclerosing agent. (C) Hemorrhoid foam sclerosing agent ids after injection treatment with polidocanol.
After the operation, the patient was provided anemia correction treatment (500 mg of intravenous iron dextran daily for 3 days, and 0.2 g of oral ferrous succinate once daily). On postoperative Day 3, the patient had no bleeding during the first bowel movement. On postoperative Day 7, a repeat blood test showed a red blood cell count of 2.91 × 1012/l and a hemoglobin level of 72 g/l. As there was no sign of bleeding, the patient was discharged. One month after surgery, the patient’s hemoglobin level reached 115 g/l, with no bleeding symptoms. Three months after surgery, the hemoglobin level returned to 139 g/l, and all the clinical symptoms were fully relieved. Iron supplements were discontinued. No complications related to the sclerotherapy occurred during the entire treatment process.
Conclusion
Hemorrhoids are a major cause of lower gastrointestinal bleeding [5]. In an anemic state, patients’ cells, tissues, and organs are in a state of ischemia and hypoxia, which heightens the risks of anesthesia and surgery, increases the likelihood of postoperative infections and mortality, and slows recovery [6, 7]. Thus, it is crucial to devise safer, more convenient, and effective treatment plans for patients with hemorrhoid-related anemia. In this case, the patient had long-term hemorrhoids with chronic bleeding and severe anemia. After thorough communication, surgical treatment was chosen. Given the patient’s severe anemia and hemodynamic instability, local infiltration anesthesia was selected to avoid hemodynamic fluctuations from anesthesia. To achieve rapid hemostasis, minimize intraoperative and postoperative bleeding, and prevent worsening anemia, the minimally invasive injection therapy was chosen.
Injection therapy for internal hemorrhoids works by injecting a sclerosing agent into the submucosal layer and hemorrhoidal mass, inducing aseptic inflammation, coagulation of the hemorrhoidal mass, vessel occlusion, and fibrotic fixation of hemorrhoidal tissue. This leads to hemorrhoid shrinkage and hemostasis [8]. Additionally, it enables quick hemostasis, and offers high reproducibility [9]; if initial sclerotherapy fails, a second treatment can be done within 2 weeks. Polidocanol, an emerging sclerosing agent, has shown significant efficacy and safety in sclerotherapy for hemorrhoids of grades I–III and their bleeding [10]. Compared to other agents, polidocanol foam has a high effectiveness rate of 94.7%–98% and a low complication rate of ~1.5% [10, 11], which make it suitable even for pediatric internal hemorrhoids [11]. It also has a local anesthetic effect [12], reducing pain during treatment.
In summary, polidocanol injection therapy for hemorrhoids is barely limited by the patient’s general condition or anesthesia method. It is ideal for patients with hemorrhoids and anemia. Its simple procedure, relatively high safety, and minimal invasiveness reduce intraoperative bleeding and postoperative anemia exacerbation. With proven short-term efficacy [13], it serves as an effective bridging therapy [14].
Conflict of interest statement
None declared.
Funding
None declared.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.



