-
PDF
- Split View
-
Views
-
Cite
Cite
Masashi Nagata, Jun Miyagi, Yoshihiko Kanai, Shinichi Yamamoto, Hiroyoshi Tsubochi, Shunsuke Endo, Regressed anterior mediastinal nodule secondary to removal of dental crowns, Journal of Surgical Case Reports, Volume 2025, Issue 10, October 2025, rjaf786, https://doi.org/10.1093/jscr/rjaf786
Close - Share Icon Share
Abstract
The causality of thymoma (TM) or thymic hyperplasia and various autoimmune diseases (AISs) remains poorly understood. To date, there have been no confirmed cases of pathologically complete regression of TM. We present a rare case of complete regression of an anterior mediastinal tumour secondary to the removal of metallic dental crowns as a treatment for metal allergy assessed to exacerbate pityriasis rubra pilaris, a rare cutaneous disease. After dental crown removal, the skin lesions improved, and the anterior mediastinal nodule (AMN) shrank rapidly through transient enlargement with a low density in the central area. Despite observation being an option, the patient preferred surgical resection and pathological examination, which revealed no remnant neoplasm. Treatment for skin conditions rarely induces complete regression of the AMN. This case highlights the potential novel link between anterior mediastinal tumours and AISs, including cutaneous disorders, and raises awareness amongst clinicians of this possible association.
Introduction
The correlation between thymoma (TM) or thymic hyperplasia (TH) and multiple autoimmune diseases (AISs), including dermatologic conditions such as oral lichen planus and pemphigus, is complex and not fully understood [1, 2]. Thymothymectomy has reportedly improved or resolved AISs, whilst AIS treatment has been associated with a reduction in thymic nodules [3, 4]. Regression of TM has been documented in cases involving tumour infarction [5], steroid therapy [6] and other treatments for AISs [7]; however, complete pathological regression has not been definitively confirmed. The underlying mechanism may involve dysregulated helper-T cells and interleukins produced by genetically altered TMs, contributing to AIS pathogenesis [1]. Nonetheless, the precise processes through which AISs contribute to TH or TM remain unclear.
We present a rare case of complete anterior mediastinal nodule (AMN) regression expected to be TM or TH secondary to dental crown removal for treating metal allergy, assessed to aggravate pityriasis rubra pilaris (PRP), a cutaneous AIS.
Case report
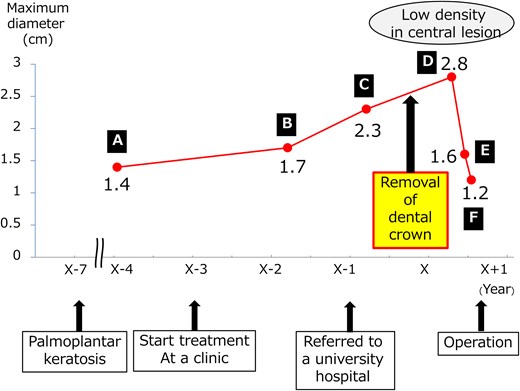
In the year X, a woman in her forties presented for the evaluation of a 2.8-cm AMN (Fig. 1d). She was a former smoker and was on medication for diabetes mellitus and hyperlipidaemia. In X-4, her physician performed computed tomography (CT) scan to exclude malignancy for uncontrolled diabetes mellitus and detected a well-circumscribed and slightly lobulated 1.4-cm AMN (Fig. 1a). Upon follow-up, the AMN had grown to 1.7 cm and 2.3 cm in X-2 (Fig. 1b) and X-1 (Fig. 1c), respectively. The AMN on CT at referral was enlarged but had blurred margins and low density. On enhanced CT performed 2 months later, the size of the AMN was 1.4 cm with a low density in the central area (Fig. 1e). 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) performed 3 months later showed the AMN measured 1.2 cm with little accumulation (Fig. 1f). FDG-PET and brain MRI revealed no metastases. There were no abnormal values for any of the laboratory tests, including tumour markers, anti-acetylcholine receptor antibody and thyroid function. We considered this change to suggest that the TM regressed spontaneously with an unclear aetiology and proposed close observation as one possible option. However, the patient opted for resection and pathological examination. Six months after referral, we performed a thoracoscopic resection of the AMN with a sufficient surgical margin. The target nodule was detected in the resected specimen. The peri- and post-operative courses were uneventful. The patient was discharged on post-operative day 6.
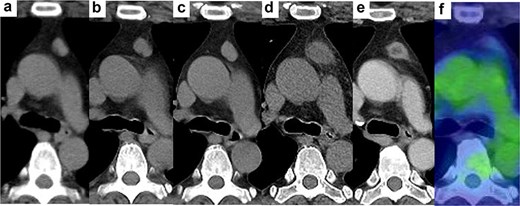
Changes in AMN on CT. (a) 4 years before visiting our hospital, (b) 2 years before, (c) 1 year before, (d) at the time of visiting our hospital, half a year after the removal of all dental crowns, (e) 2 months after visiting our hospital, (f) positron emission tomography 3 months after visiting our hospital.
The histopathological diagnosis was xanthogranulomatous inflammation and abscess with no evidence of neoplasms, including TM. Macroscopically, a 1.0-cm yellow-white nodule was observed (Fig. 2a and b). Microscopically, numerous histiocytes containing fat components were aggregated, and neutrophilic cells had accumulated with abscess formation, surrounded by a thick fibrotic capsule (Fig. 3a–c). No neoplastic cells were found. Immunohistological staining, including AE1/AE3 and p63, did not suggest the presence of TM. Only a few hollow epithelial cell structures were detected in the nodule, consistent with normal thymic tissue (Fig. 3d).
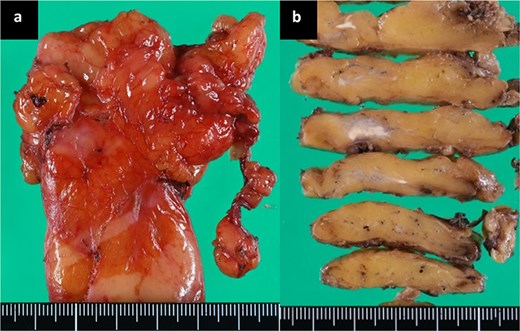
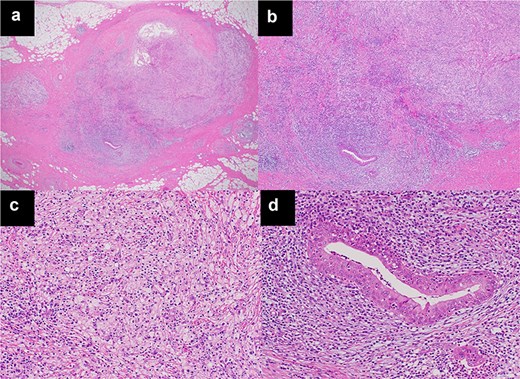
Microscopic findings using haematoxylin and eosin staining. (a) (×20), (b) (×50), (c) (×200). The sections show xanthogranulomatous inflammation and abscesses with no evidence of neoplasms, including TM. (d) Only a few hollow structures of epithelial cells, suggesting normal thymic tissue in the nodule, were detected (×200).
Post-operatively, we obtained her detailed PRP history. In X-7, the patient developed palmoplantar keratotic erythema. Treatment began at a dermatology clinic in X-3 (Fig. 4a and b). Despite topical medication and phototherapy, lesions persisted. In X-1, she was referred to a university hospital dermatology department. Incisional biopsy confirmed PRP. Patch testing indicated metal hypersensitivity. Consequently, 6 months before referral, she had all metal dental crowns, placed during youth, removed. Palmar lesions improved substantially immediately after removal; however, plantar lesions showed minimal change (Fig. 4c and d). The timeline appears in Fig. 5. She never received systemic steroids or immunosuppressive therapy.
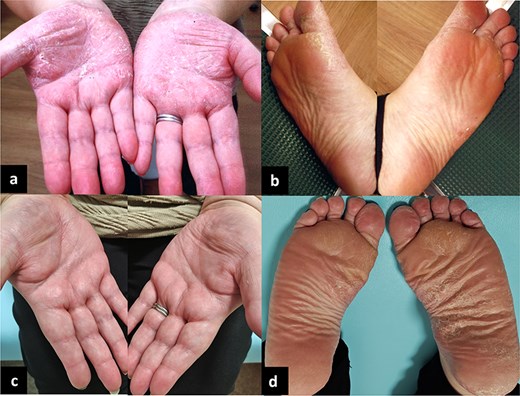
Palmoplantar lesions of her skin disease, PRP. (a, b) 3 years before visiting our hospital. (c, d) At the time of visiting our hospital, half a year after the removal of all dental crowns. The palmar lesions ameliorated after the removal of the dental crowns.
After surgery, the skin lesions remained unchanged. A CT scan performed 1 year later revealed no post-operative complications or recurrence. The patient was satisfied with the treatment outcome.
Discussion
In this case, AMN completely regressed following dental crown removal in a patient with PRP worsened by metal allergy. We were unable to identify AMN pathologically. Given the radiological findings and spontaneous/AIS treatment-related regression, the AMN was most compatible with a TH or low-grade TM. TH is usually characterized by diffuse symmetric enlargement on images. However, mass-like TH often mimics TM [8]. High-grade TM and thymic carcinoma can be distinguished by the degree of FDG uptake on PET imaging [9].
A similar clinical course to our case is seen in reports of complete TH regression following treatment for Graves’ disease (GD), likely due to reduced proliferation via thyroid-stimulating hormone receptors in the thymus and thyroid hormone effects on thymopoiesis [3, 4]. The two major TH subtypes are true TH (proliferation of normal thymic tissue) and lymphoid TH (proliferating lymphatic follicles in extrathymic spaces) [10]. True TH often accompanies autoimmune endocrine diseases, including GD [10]. However, lymphoid TH with GD has also been reported, which persists after achieving euthyroidism [2, 11].
PRP is a rare papulosquamous inflammatory dermatosis often affecting the palmoplantar region [12], distinct from palmoplantar pustulosis, which is associated with metal allergy as an exacerbating factor. Whilst metal allergy is not implicated in PRP, the dermatologist likely conducted allergy testing due to the clinical similarity of palmoplantar dermatoses. Although PRP aetiology remains poorly understood, recent studies reported the involvement of autoimmune factors, including helper T cells and interleukin dysregulation, in PRP and palmoplantar pustulosis onset [12, 13]. Although coexistence of PRP and TM or TH remains undocumented, this is considered plausible. In our case, RPR and accelerator metal allergy likely promoted AMN formation. Further studies are required to evaluate the mechanisms.
We learned of our patient’s PRP after AMN treatment. This demonstrates insufficient interest in the relationship between AMN and AISs, including integumentary diseases. Clinicians, particularly thoracic surgeons, should consider this association. Thymic carcinoma reportedly regresses spontaneously [14] and is accompanied by AISs [15]. As noted above, TH may not reduce despite treatment for GD [11]. If AMN shows atypical changes and potential malignancy, resection for pathological confirmation and treatment is warranted.
In conclusion, we report an exceptional case of complete AMN regression following dental crown removal, performed as part of PRP treatment in a patient with metal allergy. This case suggests a possible causal link between AMN and AISs, including skin disorders.
Acknowledgements
We thank Dr. Kayoko Higuchi, a pathologist at Okinawa Kyodo Hospital, for diagnostic pathology.
Conflict of interest statement
None declared.
Funding
None declared.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
Ethics statement
This case report complied with the Declaration of Helsinki (revised in Fortaleza, Brazil, October 2013) and was approved by the Institutional Review Board of our hospital (approval number: 2025–02). Written informed consent was obtained from all patients.



