-
PDF
- Split View
-
Views
-
Cite
Cite
Saad Assila, Mouna Khmou, Youssef Mahdi, Basma El Khannoussi, Mesonephric-like adenocarcinoma of the ovary: a case study, Journal of Surgical Case Reports, Volume 2025, Issue 1, January 2025, rjaf025, https://doi.org/10.1093/jscr/rjaf025
Close - Share Icon Share
Abstract
Mesonephric-like adenocarcinoma (MLA) is a rare and newly recognized subtype of ovarian and endometrial carcinomas, introduced in the 2020 World Health Organization Classification. This tumor likely originates from Müllerian-derived tissues and often mimics more common ovarian cancers, leading to frequent misdiagnosis. This case study details a 36-year-old woman who presented with urinary symptoms following a hysterectomy. Imaging revealed a significant left ovarian mass, initially misdiagnosed as carcinosarcoma. Pathological evaluation ultimately confirmed MLA, characterized by diverse architectural patterns and specific immunohistochemical markers. The patient underwent chemotherapy due to the locally advanced disease. This case highlights the diagnostic challenges of MLA and emphasizes the need for awareness among clinicians to prevent misdiagnosis. Given its aggressive nature and tendency to early recurrence, further research is essential for establishing standardized diagnostic criteria and treatment protocols for this rare malignancy.
Introduction
The 2020 World Health Organization Classification of Tumors of the Female Reproductive System introduced mesonephric-like adenocarcinoma (MLA) as a newly recognized and rare subtype of ovarian and endometrial carcinomas [1]. MLA most likely represents a Müllerian-derived neoplasm exhibiting differentiation along mesonephric lines [2]. The clinical presentations associated with MLA of the ovary resemble those observed in other more common subtypes of ovarian cancer [1]. The morphological features, immunohistochemical profile, and molecular characteristics of MLA are analogous to those observed in mesonephric adenocarcinoma (MA) [1, 3]. Nonetheless, the tumor tissue in MLA does not contain any remnants of mesonephric ducts in its surrounding area [4]. Furthermore, due to its rarity and overlapping features with more common ovarian tumors, MLA is frequently misdiagnosed leading to inappropriate treatment and a delay in diagnosis [5]. At present, its treatment approach involves primary surgical resection typically followed by adjuvant chemotherapy [1]. This case study article adds to the limited body of literature on this rare aggressive and diagnostically challenging tumor, which can help prevent misdiagnosis.
Case report
A 36-year-old woman was admitted to the hospital on December 2022 with burning micturition and pollakiuria that appeared 2 months after a subtotal interannexal hysterectomy for a uterine leiomyoma. The patient had a history of three Pfannenstiel myomectomies. Considering this urinary symptomatology, a computed tomography (CT) scan was performed revealing a voluminous 158 mm mass along the left abdominopelvic axis suggestive of a left ovarian tumor together with peritoneal carcinomatosis. In January 2023, a left ovarian mass was found during an exploratory laparotomy and was biopsied. An outpatient basis initial reading diagnosed a carcinosarcoma. Contacted for a second reading, our pathological anatomy laboratory at the institute concluded on tumor proliferation with a mixture of architectural patterns, notably glandular, tubular cordonal, and spindled/solid (Fig. 1A–C). Certain glands and tubules contained eosinophilic colloid-like material (Fig. 1A). Tumor cells were medium-to-large in size, with eosinophilic cytoplasm and vesicular, anisokaryotic nuclei, sometimes highly atypical with nuclear overlap, prominent nucleoli, and increased mitotic activity (Fig. 1D). The cells were positive for CKAE1/AE3, EMA, CD10, and TTF1 and negative for GATA3 (Fig. 2A and B). Thus, the morphological appearance and immunohistochemical profile were compatible with an ovarian MLA. In a multidisciplinary consultation meeting, the locally advanced tumor with associated carcinomatosis deemed impossible a surgical treatment. The patient was treated with six cycles of Paclitaxel-Carboplatin and one cycle of Bevacizumab. During her follow-up care, the last CT scan showcased stable lesion characteristics on July 2024.
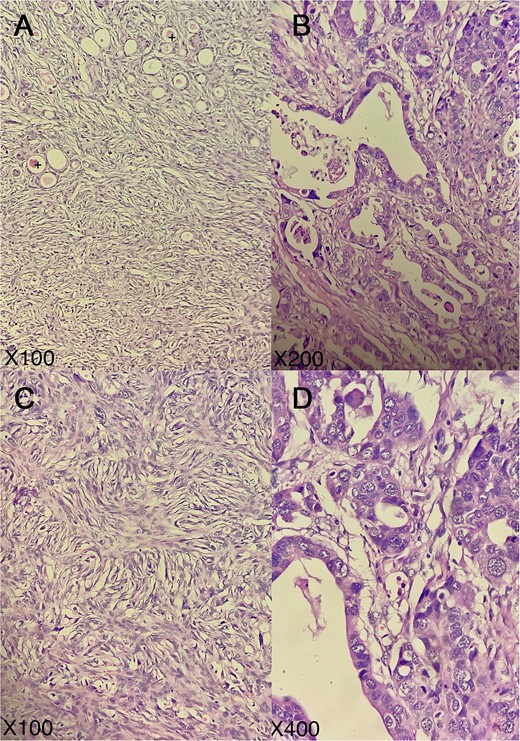
Representative micrographs of the tumor; tumor exhibits a combination of architectural patterns (A), notably glandular, tubular cordonal (B), and spindled/solid (C); certain glands and tubules contained eosinophilic colloid-like material (+); tumor cells are medium-to-large in size, possessing eosinophilic cytoplasm and vesicular, anisokaryotic nuclei, sometimes highly atypical with nuclear overlap, prominent nucleoli, and increased mitotic activity (D); (hematoxylin–eosin; A, C: × 100, B: × 200, D: × 400).
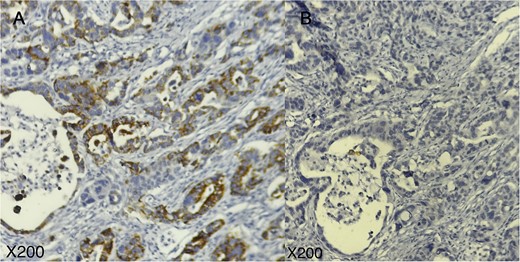
Immunohistochemical profile of the tumor; tumor cells are positive for TTF1 (A) and negative for GATA3 (B); (A, B: × 200).
Discussion
MLAs are rare neoplasms originating in the uterine corpus and ovary. They were first identified and described in 2016 by McFarland et al. [6]. The histological origin of MLA remains controversial. While MLAs exhibit morphological and immunohistochemical features analogous to those observed in MA suggesting mesonephric origins, a potential Müllerian origin with subsequent mesonephric transdifferentiation is supported by the absence of mesonephric remnants within the tumor and the association of MLAs with Müllerian lesions (i.e. endometriosis, cystadenomas, adenofibromas, borderline tumors, and low-grade serous carcinomas) [6–8].
Clinically, MLAs of the ovary closely resemble other ovarian cancers. Patients may be asymptomatic in the early stages, while late-stage symptoms include chronic pelvic pain, abnormal uterine bleeding (especially postmenopausal irregular bleeding), and/or abdominal distension [1].
Macroscopically, MLA tumors do not exhibit a distinctive appearance. They are typically unilateral and vary in size from 4 to 32 cm. Their cut surface can be solid or solid and cystic, displaying a grayish-white or yellowish-tan appearance [6]. Histologically, MLAs are identical to MA in other areas of the female genital tract. Nonetheless, they lack associated mesonephric remnants/hyperplasia [1, 4]. Microscopic features include tubular, glandular (pseudoendometrioid), slit-like, retiform, papillary, and solid patterns with the tumor usually exhibiting two or more patterns [1, 4, 6]. Eosinophilic colloid-like material is frequently observed in the lumen of the tubules [1, 6]. Neoplastic cells may be flat, cuboidal, and columnar with eosinophilic cytoplasm. The nuclei have dense or vesicular chromatin with inconspicuous nucleoli. They usually show crowding and nuclear grooves [1, 4, 6]. No squamous and mucinous differentiation is seen [1, 4, 6]. Endometriosis is often associated with MLAs [4, 6].
Immunohistochemically, MLAs are characterized by positivity for GATA3, TTF1, calretinin, and CD10, wild-type p53 expression, and negativity for ER/PR [1, 6]. GATA3 and/or TTF1 are regarded as MLA markers [5]. Nonetheless, GATA3 is considered less reliable as a marker in the presence of a spindle cell component as observed in our patient [9]. Molecularly, MLAs exhibit activating mutations in KRAS and alterations in chromosome 1 (1q gain with or without 1p loss) [4, 10]. Unlike MA, approximately half of MLA also contain PIK3CA mutations [4, 5, 10], but exhibit neither microsatellite instability nor PTEN mutation/deletion [4, 10].
The differential diagnosis of ovarian MLA is complex, especially on biopsy material, because of its overlapping histological features with other ovarian neoplasms. Endometrioid carcinoma is distinguished from MLA by the lack of numerous architectural patterns; the presence of squamous, mucinous, and/or sertoliform differentiation; the positivity for ER/PR; and the negativity for GATA3 and TTF1 [4, 9]. High-grade serous carcinoma is characterized by high-grade nuclear atypia, intense mitotic activity with numerous atypical mitoses throughout the tumor; uniform nuclear WT1, and mutant p53 type expression besides diffuse p16 immunostaining [4, 9]. Clear-cell carcinoma exhibits abundant clear-to-eosinophilic cytoplasm, distinct cell borders, and hyalinized or myxoid stroma; and is characterized by TTF1 and GATA3 negativity, unlike MLA [9]. Despite similar architectural patterns, Wolffian tumor lacks expression of TTF1 and GATA3, characteristic of MLA [9].
MLAs frequently appear at advanced stages and demonstrate early recurrence resulting in a poor prognosis [11]. While its treatment lacks consensus, it often involves a total hysterectomy with bilateral adnexectomy, omentectomy, and pelvic lymphadenectomy, followed by combination chemotherapy, consisting of carboplatin and paclitaxel [1]. For our patient, given the locally advanced malignancy, the treatment was chemotherapy from the outset.
Conclusion
Ovarian MLA is an easily misdiagnosed neoplasm given its rarity and histological overlapping aspects with other ovarian malignancies. Nevertheless, in the face of coexisting diverse histological patterns, MLA should be dismissed by TTF1/GATA3 immunohistochemistry. Additional studies of MLA are expected to facilitate diagnosis and standardize treatment.
Author contributions
All authors read and approved the final manuscript.
Conflict of interest statement
The authors declare that they have no competing interests.
Funding
No funding source was needed.
Data availability
Not applicable.
Consent
Informed consent was obtained from the patient.
Guarantor
The corresponding author is the guarantor.



