-
PDF
- Split View
-
Views
-
Cite
Cite
Shangdao Lai, Tao Yuan, Bing Huang, Jiongfeng Liu, Yanzhong Chen, Zhiqiang Huang, Yuquan Liu, Feiran Lai, CT-guided radiofrequency neurotomy (RFN) of bilateral T3–4 sympathetic chain combined with bilateral L3 sympathetic ganglion in patient with palmar hyperhidrosis, Journal of Surgical Case Reports, Volume 2025, Issue 1, January 2025, rjae808, https://doi.org/10.1093/jscr/rjae808
Close - Share Icon Share
Abstract
Palmoplantar hyperhidrosis is a functional disease with an unknown pathogenesis, making it challenging to find a lasting and effective treatment. This article reports a case of a 43-year-old patient with palmoplantar hyperhidrosis treated with computed tomography (CT)-guided radiofrequency neurotomy (RFN) of bilateral T3–4 sympathetic chain combined with bilateral L3 sympathetic ganglion. The optimal puncture level and skin entry point were selected, and measurements were taken using a CT tool to determine needle depth, angle, and distance from the midline. A sympathetic needle was inserted through the T4 intercostal space to the outer side of the T4 rib head. The needle position was adjusted to achieve a tissue resistance, confirmed through sensory, motor stimulation, and three-dimensional reconstruction. RFN was performed, and this process was repeated for 1–2 cycles. Subsequently, a similar procedure was performed at the L3 sympathetic ganglion under CT guidance, resulting in improved sweating symptoms in the patient’s hands and feet.
Case description
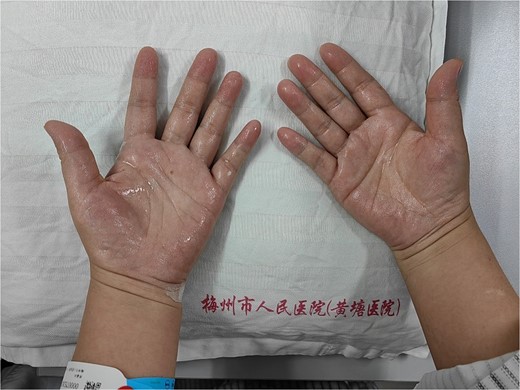
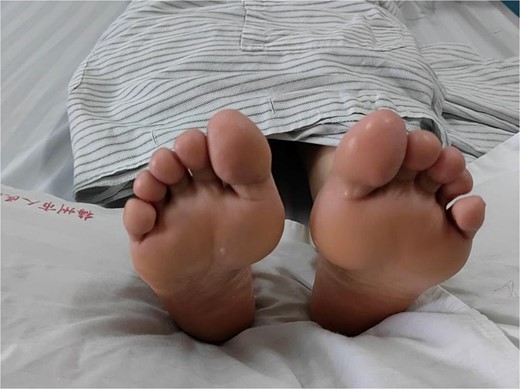
This case involves a 43-year-old woman who presented to the pain clinic at Meizhou People’s Hospital with abnormally enhanced sweating on both sides of her hands and feet, which had persisted for 40 years. Sweat drops fell down after 5 min of rest, accompanied by increased axillary sweating. The palms and feet were moist and could be left to drip sweat for 5 min (Figs. 1 and 2).
Surgical procedure
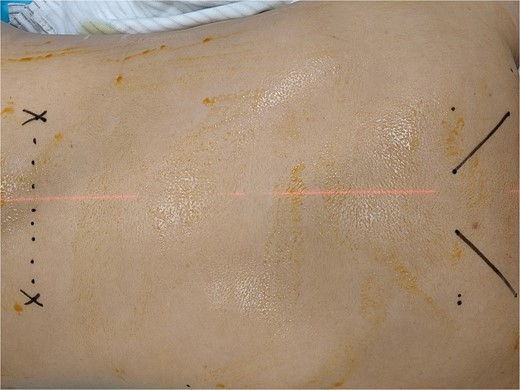
The patient underwent routine fasting for 4 h, and the infusion channel was opened. Upon admission to the CT operating room, the patient was positioned prone on the CT table with soft pillows under the chest. Vital signs, including blood pressure, heart rate, palm and foot temperatures, and pulse perfusion index, were monitored. The T4 intervertebral space was located using a localization image. After plain scanning, the optimal puncture level and skin puncture point were selected. The proposed depth of needle entry, angle, and distance of the entry point from the midline were measured using a CT tool ruler (Fig. 3).
The position of the needle tip was adjusted until the resistance of the tissue around the tip of the test electrode ranged between 150 and 250 Ω. After confirmation through three-dimensional reconstruction (Fig. 4), the needle was withdrawn without encountering blood, liquid, or gas. The temperature was set to 80–95°C, and thermal coagulation was maintained for 120 s. A total of 1–2 cycles of radiofrequency thermocoagulation were performed (Fig. 5).
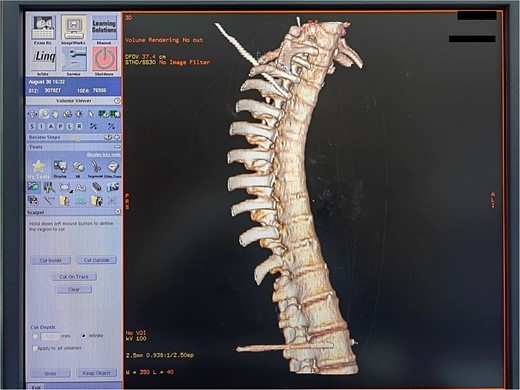
Three-dimensional reconstruction of a successful thoracic and lumbar sympathetic ganglion puncture.
1–2 cycles of radiofrequency thermocoagulation of thoracic and lumbar sympathetic nerves.
Following the withdrawal of the needle and a subsequent CT scan, no pneumothorax was observed in the lung window, leading to the conclusion of the treatment. Subsequently, RFN of the thoracic sympathetic was performed. Under CT guidance, L3 sympathetic ganglion RFN was initiated. The patient assumed a prone position in the CT room with a soft pillow under the abdomen, and vital signs, such as blood pressure and heart rate, were monitored. The L2–3 vertebral body was identified through CT localization, and after a plain scan, the optimal puncture level and skin puncture point were selected. The proposed depth of needle penetration at the puncture point, as well as its angle and distance from the midline, were measured using a CT tool ruler. The angle and relative distance between the CT bed and the rack displayed at that level were also recorded (Fig. 6).
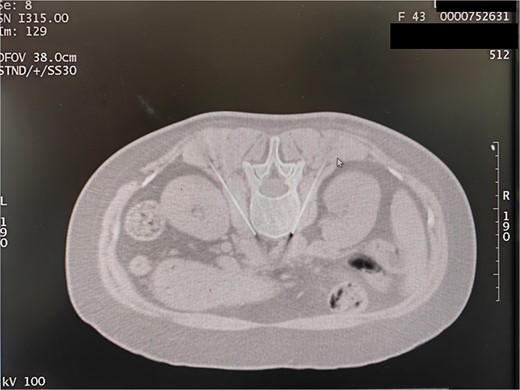
Following local anesthesia at the selected puncture point, a CT-guided puncture to the L3 sympathetic target point was executed in accordance with the proposed angle and depth. The position of the needle tip was adjusted to assess the resistance of the tissue around the electrode tip, aiming for a range of 150–250 Ω. Subsequently, the accuracy of the radiofrequency tip position was confirmed through sensory and motor electrical stimulation and three-dimensional reconstruction (Fig. 7).

Three-dimensional reconstruction of a successful lumbar sympathetic ganglion puncture.
The patient’s foot temperature increased, and there were no reported numbness or limb activity disorders. After repositioning the needle and conducting a CT scan, observation of the lung window revealed no occurrence of pneumothorax. Following the conclusion of the treatment, the radiofrequency electrodes were withdrawn, and after removing the needle, the puncture point was localized and covered with a band-aid. Throughout the procedure, the patient’s vital signs remained stable.
Discussion
Primary hyperhidrosis (PH) is a sympathetic disease that commonly affects the palms, soles, axillae, or craniofacial region, typically beginning in childhood or adolescence [1]. The specific pathogenesis of PH has not been clearly defined but is often genetically related, attributed to sympathetic hyperexcitability leading to excessive sweat gland secretion [2]. This condition negatively impacts emotional, physical, and psychological well-being, with the combination of sweaty palms and feet known as palmoplantar hyperhidrosis [3].
Currently, various treatments exist for palmoplantar hyperhidrosis. However, a permanent cure can be achieved through surgical treatment. Surgical options include thoracoscopic sympathetic chain cutting and CT-guided thoracic and lumbar sympathetic radiofrequency sympathectomy [4]. While laparoscopic lumbar sympathectomy can address foot sweating, the technology is still in its early stages, leading to numerous complications [5]. Laparoscopic lumbar sympathectomy can address sole sweating, but the technique is still immature and associated with more complications [6]. RFN is gradually gaining recognition as a safer, more effective, and minimally invasive treatment [7]. However, there are limited case studies on the use of RFN for treating palmoplantar hyperhidrosis.
Here, we investigated the effectiveness and safety of CT-guided percutaneous puncture thoracolumbar sympathetic radiofrequency ablation in the treatment of hand and foot hyperhidrosis. Under CT guidance, the radiofrequency needle was accurately inserted to the position of the chest and lumbar sympathetic meridian chain, and radiofrequency ablation was carried out respectively. Hand sweat glands are primarily regulated by the thoracic sympathetic nerve. Currently, it has been confirmed that T2–4, T2–3, and T3–4 ablation can effectively treat hand sweating. The choice of T3–4 radiofrequency ablation can reduce the incidence of postoperative compensatory hyperhidrosis [8–10]. Following radiofrequency ablation of the thoracic sympathetic nerve, bilateral L3 sympathetic ganglion radiofrequency ablation is performed, which also demonstrates a certain effect on foot sweating [11, 12]. CT-guided radiofrequency ablation of sympathetic ganglia to treat hand and foot hyperhidrosis requires only local anesthesia infiltration, providing long-lasting curative effects and safe surgical procedures [13]. Under CT guidance, three-dimensional reconstruction is conducted by measuring the depth and angle of the puncture to minimize puncture errors and reduce complications [14, 15].
In conclusion, CT-guided RFN of bilateral T3–4 sympathetic chain with L3 sympathetic ganglion proves to be an effective treatment for palmoplantar hyperhidrosis. This approach is not only minimally invasive but also associated with fewer complications, making it a promising avenue for further research and application.
Conflict of interest statement
None.
Funding
This work was supported by the Scientific Research and Cultivation Project of Meizhou People’s Hospital (No. PY-C2019023).



