-
PDF
- Split View
-
Views
-
Cite
Cite
Kristali Ylli, Jowhara AlQahtani, Ihab Hraishawi, Thomas Murphy, VACStent closure of oesophageal defects by covered stent and endoscopic vacuum therapy: initial use and clinical outcomes, Journal of Surgical Case Reports, Volume 2025, Issue 1, January 2025, rjae804, https://doi.org/10.1093/jscr/rjae804
Close - Share Icon Share
Abstract
Endoscopic management of transmural oesophageal defects following esophagectomy or spontaneous perforations, such as Boerhaave’s syndrome, is often complicated by stent migration and luminal occlusion [1]. The Vacuum-Assisted Closure (VAC) stent, which integrates a covered stent with endoscopic vacuum therapy, aims to address these issues by providing functional drainage and promoting wound healing [2]. This case series presents our initial experience with VACStent therapy in four patients treated between February 2023 and April 2024. Two patients had staple line defects post-esophagectomy, and two had Boerhaave’s syndrome. Treatment involved stent placement under general anaesthesia, followed by evaluations and scheduled stent exchanges every 6 days. All patients achieved successful defect closure, with no procedural complications noted. Three patients required one stent application, while one needed two applications. VACStent therapy appears to be a safe and effective treatment for transmural oesophageal defects, potentially establishing a new standard of care.
Introduction
Endoscopic management of transmural oesophageal defects, whether following esophagectomy or due to spontaneous perforations such as Boerhaave's syndrome, faces challenges including stent migration and luminal occlusion [3]. The Vacuum-Assisted Closure (VAC) stent presents a novel solution, integrating a fully covered intestinal stent within a polyurethane sponge cylinder to combine the benefits of endoscopic vacuum therapy (EVT) and functional drainage [4]. This innovative design aims to enhance defect drainage and promote wound healing. In this case series, we share our initial experiences utilizing VACstent therapy [5].
The VAC stent is an innovative solution that integrates a fully covered intestinal stent with a polyurethane sponge cylinder to provide continuous drainage and promote wound healing [6]. This case series presents our initial clinical experience with the VAC stent in managing oesophageal defects. Between February 2023 and April 2024, four patients—two with staple line defects post-esophagectomy and two with Boerhaave’s syndrome—were treated using the VAC stent (Table 1). The treatment involved initial stent placement under general anaesthesia, followed by endoscopic evaluations and scheduled stent exchanges every 6 days. Our findings indicate successful defect closure in all patients with no procedural complications, suggesting that the VAC stent may offer a safer and more effective alternative to traditional therapies in the management of oesophageal defects.
| Variable . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . |
|---|---|---|---|---|
| Age (years) | 62 | 89 | 59 | 49 |
| Gender | Male | Male | Male | Female |
| Diagnosis | Adenocarcinoma of the oesophagus | Boerhaave syndrome | Boerhaave syndrome | Adenocarcinoma of the oesophagus |
| Initial therapy | MIO | Conservative | Conservative | MIO |
| ASA score | 3 | 4 | 4 | 2 |
| Complications | Anastomotic leakage | Oesophageal perforation | Oesophageal perforation | Anastomotic leakage |
| VACStent therapies | 2 | 1 | 1 | 1 |
| Total duration of VAC-Stent therapy (days) | 12 | 6 | 6 | 6 |
| Hospitalization duration (days) | 25 | 24 | 11 | 82 |
| Variable . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . |
|---|---|---|---|---|
| Age (years) | 62 | 89 | 59 | 49 |
| Gender | Male | Male | Male | Female |
| Diagnosis | Adenocarcinoma of the oesophagus | Boerhaave syndrome | Boerhaave syndrome | Adenocarcinoma of the oesophagus |
| Initial therapy | MIO | Conservative | Conservative | MIO |
| ASA score | 3 | 4 | 4 | 2 |
| Complications | Anastomotic leakage | Oesophageal perforation | Oesophageal perforation | Anastomotic leakage |
| VACStent therapies | 2 | 1 | 1 | 1 |
| Total duration of VAC-Stent therapy (days) | 12 | 6 | 6 | 6 |
| Hospitalization duration (days) | 25 | 24 | 11 | 82 |
| Variable . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . |
|---|---|---|---|---|
| Age (years) | 62 | 89 | 59 | 49 |
| Gender | Male | Male | Male | Female |
| Diagnosis | Adenocarcinoma of the oesophagus | Boerhaave syndrome | Boerhaave syndrome | Adenocarcinoma of the oesophagus |
| Initial therapy | MIO | Conservative | Conservative | MIO |
| ASA score | 3 | 4 | 4 | 2 |
| Complications | Anastomotic leakage | Oesophageal perforation | Oesophageal perforation | Anastomotic leakage |
| VACStent therapies | 2 | 1 | 1 | 1 |
| Total duration of VAC-Stent therapy (days) | 12 | 6 | 6 | 6 |
| Hospitalization duration (days) | 25 | 24 | 11 | 82 |
| Variable . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . |
|---|---|---|---|---|
| Age (years) | 62 | 89 | 59 | 49 |
| Gender | Male | Male | Male | Female |
| Diagnosis | Adenocarcinoma of the oesophagus | Boerhaave syndrome | Boerhaave syndrome | Adenocarcinoma of the oesophagus |
| Initial therapy | MIO | Conservative | Conservative | MIO |
| ASA score | 3 | 4 | 4 | 2 |
| Complications | Anastomotic leakage | Oesophageal perforation | Oesophageal perforation | Anastomotic leakage |
| VACStent therapies | 2 | 1 | 1 | 1 |
| Total duration of VAC-Stent therapy (days) | 12 | 6 | 6 | 6 |
| Hospitalization duration (days) | 25 | 24 | 11 | 82 |
Series of case reports
First case
A 62-year-old male presented with ypT3N2R0 invasive adenocarcinoma of the lower thoracic oesophagus, characterized by poorly differentiated mixed intestinal and diffuse types, with mucinous features and signet ring cells. The patient had suspicious lympho-vascular invasion, negative perineural invasion, clear surgical margins, and 4 out of 15 lymph nodes positive for metastatic carcinoma with multifocal extra-nodal extension. He underwent six cycles of neoadjuvant 5-Fluorouracil, Leucovorin, Oxaliplatin, and Docetaxel chemotherapy before an elective minimally invasive esophagectomy (MIO).
Postoperatively, the patient was admitted to the ICU and initially recovered well, progressing with an increased diet. However, on postoperative day 7, he developed chest pain, shortness of breath, decreased exercise tolerance, and desaturations. A computed tomography pulmonary angiography was performed, and the patient was started on Tazocin, given analgesia, and underwent a gastrografin swallow, which revealed an anastomotic leak.
The patient was treated with antibiotics and VACStent insertions, requiring two stent placements (Fig. 1a). By the second stent removal, the anastomotic leak had closed (Fig. 1b). He experienced an episode of acute kidney injury managed with fluids. By postoperative day 24, the leak had resolved, the stent and porta Cath were removed, and the patient was discharged with normal blood results and a follow-up scheduled. The patient continued to manage his overall health with regular follow-ups.
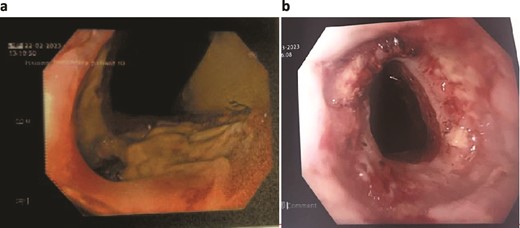
Patient One: (a) Before VACStent insertion, showing the oesophageal defect. (b) After VACStent treatment, demonstrating successful defect closure.
Second case
A 89-year-old male, diagnosed with Boerhaave syndrome and oesophageal perforation was transferred from one hospital to another on day 13 post-diagnosis. Initial diagnostic tests, including a gastrografin swallow, identified a defect in the distal left lateral wall of the oesophagus, measuring ˂1 cm with an extraluminal component of at least 5 cm (Fig. 2a).
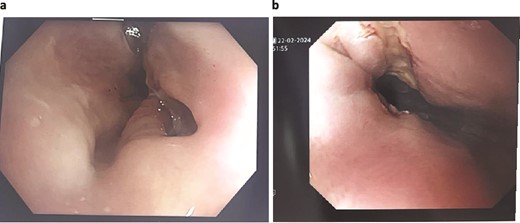
Patient Two: (a) Before VACStent insertion, showing the oesophageal defect. (b) After VACStent treatment, demonstrating successful defect closure.
On day 14 post-diagnosis, the patient underwent an oesophago-gastro-duodenoscopy (OGD) and VACStent insertion, which was kept in place for 6 days. A CT thorax on day 16 post-diagnosis confirmed proper placement of the VACStent with no ongoing leak (Fig. 2b). The patient underwent ultrasound-guided drainage for a left pleural effusion on day 17 post-diagnosis and remained nil by mouth. Subsequent imaging showed a decrease in the oesophageal defect size, and by day 22 post-diagnosis, the patient was advised to start a soft moist diet, which was well tolerated.
Throughout the admission, the patient received comprehensive care, including reviews by respiratory, dietetics, occupational therapy, physiotherapy, and geriatric teams. The patient was treated for Clostridium difficile and other infections, with antibiotics gradually de-escalated. By day 30 post-diagnosis, the patient was transferred to a convalescent centre with all tubes and lines removed, marking significant improvement and readiness for rehabilitation.
Third case
A 59-year-old male was acutely transferred from one hospital to another on day 1 post-diagnosis of oesophageal perforation. A CT scan demonstrated a hydropneumothorax with contrast draining into the left pleural space, and two chest tubes were in situ.
On day 2 post-diagnosis, the patient underwent an OGD, VACStent insertion, and ultrasound-guided pleural drain placement. The endoscopy revealed a 2 cm linear defect in the lower oesophagus just above the gastroesophageal junction (Fig. 3), and the VACStent was connected to 125 mmHg of negative pressure, confirming appropriate positioning. The patient tolerated the procedure well without intraoperative complications and was transferred to the ICU intubated.
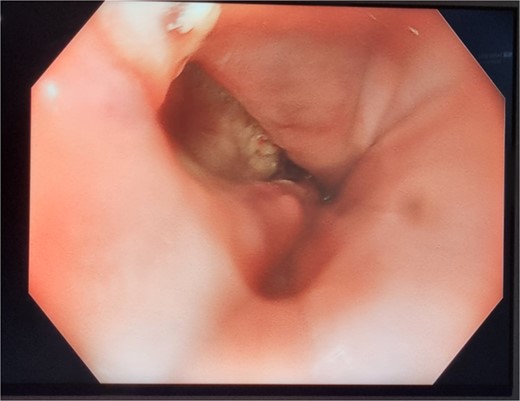
Patient Three: Before VACStent insertion, showing the oesophageal defect.
Despite initial improvements, the patient developed a left-sided empyema and was referred for decortication. On day 11 post-diagnosis, the patient underwent left-sided VATS decortication and washout. Histology confirmed inflammation consistent with oesophageal perforation. The patient gradually improved postoperatively, with a recovery supported by multidisciplinary care. The patient was discharged with ongoing follow-up and support.
Fourth case
A 49-year-old-female diagnosed with stage one moderately differentiated adenocarcinoma of the distal oesophagus (CT2 N0M0), presented with a background of scleroderma (CREST) and psoriatic arthritis. The patient was admitted for a planned MIO, which initially went well, and was transferred to ward-level care on postoperative day 5.
Unfortunately, the patient developed respiratory sepsis and a small bowel obstruction, requiring ICU readmission and intubation. An exploratory laparotomy on postoperative day 5 showed no evidence of ischemia or perforation. A subsequent OGD on postoperative day 8 revealed a 20% staple line defect (Fig. 4a), leading to VACStent and right chest drain insertion.
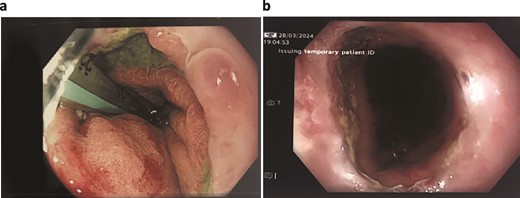
Patient Four: (a) Before VACStent insertion, showing the oesophageal defect. (b) After VACStent treatment, demonstrating successful defect closure.
Following the removal of the VACStent on postoperative day 13 (Fig. 4b), the patient underwent a prolonged antibiotic course and received dietician support for jejunal feedings. Despite complications, including unsuccessful extubation and bilateral pleural effusions, the patient gradually improved with comprehensive care. A drain was placed on postoperative day 23 to address chest wall collections, and the patient was weaned off the tracheostomy by day 31. The patient continued to recover, managed hypertension, and was discharged home on day 82, with various supports in place and a follow-up scheduled in the clinic.
Discussion
The management of transmural oesophageal defects remains a significant challenge in clinical practice due to the associated high morbidity and potential for life-threatening complications [7]. Traditional treatment methods, such as surgical repair, covered stents, and EVT, have varying degrees of success but are often limited by issues such as stent migration, luminal occlusion, and inadequate wound healing [6, 7]. This case series highlights the use of the VAC stent, an innovative approach combining the mechanical support of a covered stent with the therapeutic benefits of EVT, to address these challenges in four patients with complex oesophageal defects.
Case outcomes and implications
In the first case, a 62-year-old male with a history of invasive adenocarcinoma and an anastomotic leak post-esophagectomy demonstrated the effectiveness of the VACStent in achieving defect closure without significant complications. Despite the complexity of the patient’s condition, including acute kidney injury managed during the treatment, the VACStent facilitated successful healing. This outcome underscores the potential of VACStent therapy to manage postoperative leaks, which are notoriously difficult to treat with traditional methods.
The second case involved a patient with Boerhaave syndrome, a spontaneous oesophageal perforation that typically presents a high risk for morbidity. The VACStent not only sealed the defect but also allowed for effective drainage and resolution of associated pleural effusions. The patient’s comprehensive care included various interventions for infection control and nutritional support, highlighting the multidisciplinary approach necessary for managing such severe cases. The positive outcome in this case illustrates the VACStent’s role in promoting recovery in patients with spontaneous perforations.
The third case, a 59-year-old male with an oesophageal perforation complicated by hydropneumothorax, further demonstrates the versatility of the VACStent. The patient’s successful recovery after VACStent insertion, pleural drain placement, and subsequent VATS decortication underscores the stent’s utility in complex scenarios involving both the oesophagus and adjacent thoracic structures. This case emphasizes the importance of combining endoscopic techniques with surgical interventions when necessary, to optimize patient outcomes.
In the fourth case, a patient with stage one adenocarcinoma who developed postoperative complications, including respiratory sepsis and a small bowel obstruction, benefited from the VACStent. The management of a staple line defect using the VACStent, alongside comprehensive supportive care, led to gradual improvement and eventual discharge. This case highlights the VACStent’s role in managing not only primary oesophageal defects but also complications arising from complex surgical histories.
Advantages of VACStent therapy
The integration of the covered stent with VAC in the VACStent offers several advantages over traditional treatments [8]. First, the continuous negative pressure provided by the vacuum promotes granulation tissue formation and effective drainage of the defect site, reducing the risk of infection and facilitating faster healing. Second, the covered stent component prevents migration, a common complication with conventional stents, ensuring that the therapeutic effects are localized to the defect site [9].
Additionally, the VACStent allows for scheduled evaluations and stent exchanges, providing opportunities to monitor healing progress and make timely adjustments to the treatment plan [4]. This adaptability is particularly valuable in managing patients with complex medical histories and multiple comorbidities, as evidenced by the diverse complications observed in this case series [5].
Challenges and considerations
Despite the promising outcomes demonstrated in this series, several challenges remain. The management of oesophageal defects requires a tailored approach, considering the individual patient’s condition, comorbidities, and response to treatment. The use of the VACStent, while effective, necessitates a multidisciplinary team experienced in advanced endoscopic techniques and postoperative care [10].
Further research is needed to establish standardized protocols for the use of VACStent therapy, including optimal timing for stent exchanges, duration of therapy, and criteria for patient selection. Comparative studies with other endoscopic and surgical techniques are essential to determine the relative efficacy and cost-effectiveness of VACStent therapy [8].
Future directions
The promising results from this case series suggest several directions for future research. Prospective studies with larger patient cohorts are necessary to validate the findings and establish broader clinical guidelines. Additionally, exploring the use of VACStent therapy in other gastrointestinal applications, such as colorectal or gastric defects, could expand its utility in surgical practice [11].
Technological advancements in stent design and vacuum therapy may further enhance the efficacy and safety profile of the VACStent. Innovations aimed at improving stent material, reducing procedural complexity, and integrating real-time monitoring capabilities could significantly impact patient outcomes [11].
Conclusion
The use of the VAC stent in the management of transmural oesophageal defects represents a significant advancement in surgical and endoscopic therapy. This case series highlights the initial use and clinical outcomes of VACStent therapy in four patients with complex oesophageal defects, including postoperative anastomotic leaks and spontaneous perforations such as Boerhaave’s syndrome [12].
VACStent therapy appears to be a safe and effective treatment in the endoscopic management of transmural oesophageal defects. Its application is associated with impressive clinical and endoscopic outcomes compared to previous endoscopic therapies and may represent a new standard of care in the management of transmural oesophageal defects. The integration of a fully covered stent with EVT provides effective functional drainage, promotes wound healing, and mitigates complications commonly associated with traditional treatments, such as stent migration and luminal occlusion [13].
In our series, all patients achieved successful defect closure with minimal procedural complications, demonstrating the VACStent's potential to offer a safe and effective treatment option for these challenging conditions. These findings suggest that VACStent therapy may set a new standard of care in the endoscopic management of transmural oesophageal defects, combining the benefits of both stenting and EVT [14].
Further studies with larger patient cohorts and comparative analyses with other treatment modalities are necessary to establish definitive clinical guidelines and broaden the application of this innovative therapy. The promising outcomes observed in this series pave the way for continued research and refinement of VACStent technology, ultimately enhancing patient care in surgical gastroenterology [15].
Conflict of interest statement
None declared.
Funding
None declared.



