-
PDF
- Split View
-
Views
-
Cite
Cite
Akil Baker, Michelle Walcott-Bremmer, John Harriott, Derek Mitchell, Adhesive duodenal obstruction: a report of a previously undescribed pathology, Journal of Surgical Case Reports, Volume 2024, Issue 8, August 2024, rjae551, https://doi.org/10.1093/jscr/rjae551
Close - Share Icon Share
Abstract
Adhesive small bowel obstruction is thought to be a disorder limited to the jejunum and ileum. As a result, the list of aetiologies for duodenal obstruction does not include adhesions. We report the case of a patient who presented with gastric outlet obstruction (GOO), but with no lesions identified on cross-sectional imaging or endoscopy. Laparoscopy revealed duodenal adhesions as the cause of her GOO. Kockerization of the duodenum led to resolution of her symptoms. This previously undocumented finding leads us to suggest that laparoscopy should be considered in patients who have features highly suspicious for GOO, but have no cause identified on investigation.
Introduction
The duodenum is the first part of the small intestine. It begins distal to the pylorus of the stomach and becomes the jejunum at the ligament of Treitz. The fundamental difference between the duodenum and the rest of the small intestine is that the duodenum is predominantly a retroperitoneal structure.
Intra-peritoneal adhesions are the most common aetiology for small bowel obstruction [1]. The duodenum is spared from adhesive obstruction primarily because of two reasons: the retroperitoneal position provides protection from adhesions, and its fixation precludes kinking.
Obstruction of the proximal duodenum manifests as gastric outlet obstruction (GOO). The list of aetiologies for this disorder is well established [2, 3]. For adult patients, the most common causes are peptic ulceration, inflammatory strictures, and malignancy [3–5]. The investigations and management algorithms proposed for duodenal obstruction are based on these epidemiological data [6–8]. Since adhesive duodenal obstruction is not a described pathology, there is no consideration in management strategies for this particular diagnosis.
We present, to our knowledge, the first documented case of duodenal obstruction secondary to adhesions.
Case presentation
A 36-year-old woman presented to our surgical clinic seeking a second opinion. She gave a multi-year history of upper abdominal bloating, early satiety, and occasional vomiting. She had no medical illnesses, and her surgical history was significant for salpingectomy for an unruptured ectopic pregnancy ~18 years prior and diagnostic laparoscopy for endometriosis ~2 years prior. She had been investigated with abdominal ultrasound in the past; there was neither historical nor radiological evidence of cholecystitis or cholelithiasis. She gave no history suggestive of previous episodes of pancreatitis.
She had emergency admission 6 months prior with a clinical diagnosis of intestinal obstruction. At that time she had presented with significant abdominal distension, abdominal pain, and non-bilious vomiting. An abdominal CT scan was performed during her admission. This showed a dilated stomach with cut-off at the duodenum, but no causative lesion was identified (Fig. 1). She was managed conservatively and discharged, but continued to have intermittent episodes of bloating, pain, and anorexia over the intervening months, though not at a level that required admission.
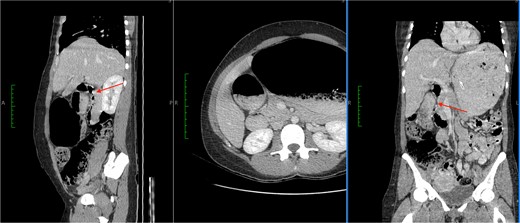
CT images showed a massively dilated stomach and duodenal cap with a point of obstruction distal to D1 of duodenum (thin arrows). No obstructing lesion was identified.
The patient had upper and lower GI endoscopy performed as an outpatient; there was evidence of mild antral gastritis, but no other pathology was identified (Fig. 2). As a result she was diagnosed as having gastroparesis and prescribed prokinetics. She gained no relief of her symptoms with this regimen.
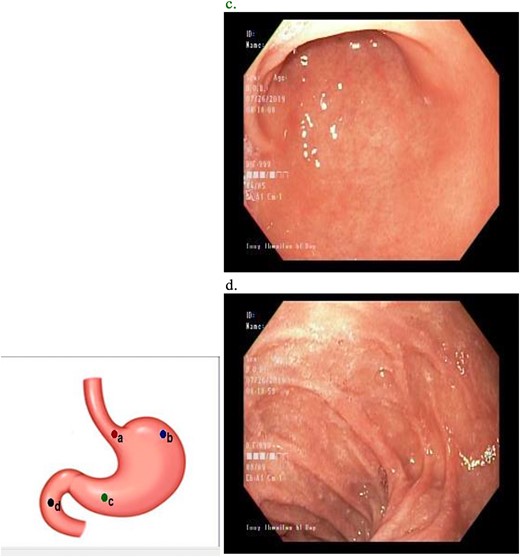
Upper GI endoscopy revealed mild antral gastritis. The duodenum was intubated and had normal mucosa.
We had a protracted conversation with the patient about differential diagnoses and the possibility that her symptoms might not be due to a surgical pathology. Having obtained informed consent, we performed a laparoscopic exploration of the upper abdomen.
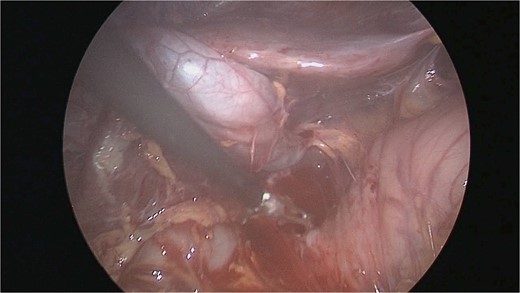
There were extensive perihepatic adhesions with involvement of the subhepatic space (Fig. 3). The initial intraoperative assessment was that this inflammation was secondary to a prior cholecystitis, but subsequent dissection revealed a pristine gallbladder (Fig. 4) with inflammatory adhesions in the pyloro-duodenal region (Fig. 5). All adhesions encasing the duodenum were lysed and the duodenum was kockerized (Fig. 6).
Extensive supra- and subhepatic adhesions were noted at laparoscopy.
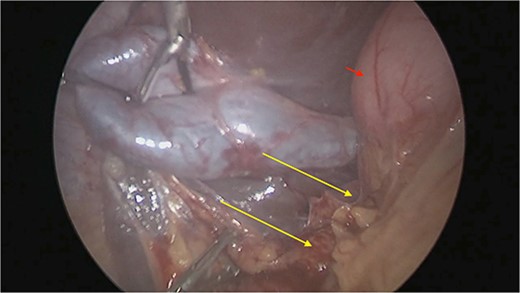
The gallbladder was dissected free of the dense adhesions. There was no evidence of previous cholecystitis. The stomach (short arrow) was spared of adhesions, but the first and second parts of the duodenum were encased (long arrows).
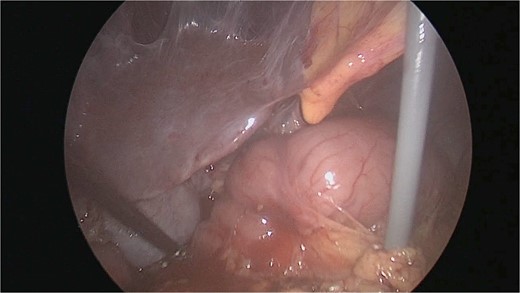
The patient was discharged on post-operative day [1] and had an uneventful recovery period. She has been symptom free for over 24 months.
Discussion
GOO is defined as mechanical obstruction in the distal stomach, pylorus, or duodenum that causes nausea, vomiting, abdominal pain, and early satiety [3]. The causes of obstruction are myriad, but can be categorized as benign or malignant [3]. The accepted investigation algorithm for patients who present with GOO is cross-sectional imaging (to identify mass lesions) followed by endoscopy (to identify luminal pathologies) [3]. Gastroparesis is diagnosed if no aetiology for GOO is found [9].
Our patient’s cross-sectional imaging identified a transition point in the duodenum, but neither intraluminal obstruction nor mass effect was identified. Adhesive duodenal obstruction is not likely to be diagnosed on imaging or endoscopy. Laparoscopy was therefore required to make the appropriate diagnosis.
We could not find any previous reference to adhesive duodenal obstruction in the surgical literature, but we expect that there have been patients with similar pathology in the past. No cause for paraduodenal scarring was identified in this patient, but it is likely that prior paraduodenal inflammation from pathologies such as pancreatitis and cholecystitis could cause a similar clinical presentation. Proper assessment of the prevalence of duodenal adhesions would require routine laparoscopy in patients diagnosed with gastroparesis, but who have features highly suspicious for GOO.
Conclusion
We present, to our knowledge, the first reported case of adhesive duodenal obstruction. This diagnosis should be considered before diagnosing a patient with functional GOO.
Conflict of interest statement
None declared.
Funding
None declared.



