-
PDF
- Split View
-
Views
-
Cite
Cite
Abdulaziz A Bazuhair, Hessa S AlSubeai, Sarah H AlKhardawi, Omar Y AlKhlaiwy, Khaldoon A Alkhums, Understanding acute chyloperitoneum in appendicitis: case report and literature review, Journal of Surgical Case Reports, Volume 2024, Issue 8, August 2024, rjae527, https://doi.org/10.1093/jscr/rjae527
Close - Share Icon Share
Abstract
Chyloperitoneum is the presence of chyle in the peritoneal cavity. This study focuses on acute chyloperitoneum, a rare condition with an unclear incidence due to limited number of reported cases in the literature. Here, we present a 24-year-old Saudi female with chyloperitoneum diagnosed intraoperatively during a laparoscopic appendectomy for acute appendicitis that was managed successfully with a low-fat diet and drainage, alongside a literature review to elucidate the condition’s pathophysiology and therapeutic strategies. A conservative management approach is recommended for acute chyloperitoneum in the context of appendicitis, this includes intraperitoneal drainage, appendectomy when needed, and careful observation. Our proposed management strategy aligns with findings from the literature review and supports conservative management as a safe and effective treatment modality for this rare condition.
Introduction
Chyloperitoneum, also known as chylous ascites, is the abnormal accumulation of chyle in the peritoneal cavity, which normally circulates through the lymphatic system.
Chyle is consisted of protein, lymphocytes, immunoglobulins, and chylomicrons. Long-chain triglycerides are broken down into monoglycerides and free fatty acids, which are then absorbed in the gut as chylomicrons [1]. The high triglyceride content in chyle gives it a milky and cloudy appearance. A triglyceride level exceeding 200 mg/dL in the ascitic fluid analysis is a key diagnostic indicator for chylous ascites [2].
Causes can be congenital, such as in cases of lymphangiomatosis, Turner syndrome, and Noonan syndrome due to lymphatic malformations, typically seen in children. They can also be acquired, more common in adults, due to factors such as lymphoma, iatrogenic injury, trauma, chronic peritoneal dialysis, or various other reasons [3]. In this study, we are focusing on acute chyloperitoneum, a condition with an unclear incidence due to a limited number of reported cases, indicating its rarity.
Management of chyle leak usually follows a step-up approach [4], starting with dietary modifications such as a high-protein, low-fat diet with medium-chain triglycerides. If no improvement is seen, pharmacological agents such as octreotide may be used, along with or without total parenteral nutrition (TPN). If these measures fail, further investigations through lymphoscintigraphy to locate the site of leakage are recommended. Subsequent management varies based on scan results, underlying causes, and available resources, with options including percutaneous embolization, transjugular intrahepatic portosystemic shunt, peritoneovenous shunting, and surgery. However, there is no standardized approach for incidentally discovered acute chyloperitoneum.
Here, we present a case of acute appendicitis with incidental finding of chyloperitoneum and literature review on the topic aim to explore the pathophysiology and management strategies for chyloperitoneum in the context of appendicitis.
Case report
A 24-year-old, unmarried, Saudi female, presented to the emergency department with severe abdominal pain for 3 days. The pain initially began at the paraumbilical area and subsequently migrated to the right lower quadrant. It was gradual in onset, with no known aggravating or relieving factors. The pain was associated with nausea and anorexia, but with no episodes of vomiting. The patient reported no changes in bowel habits, melena, hematochezia, weight loss, night sweats, fever, recent travel, or trauma. She had no history of similar previous attacks. Her menstrual cycle was regular. The patient denied smoking or alcohol use. She had no significant past medical or surgical history, and her family history was unremarkable.
Upon presentation, the patient was vitally stable except for a low-grade fever (37.8°C). Her abdominal examination revealed tenderness in the paraumbilical and right lower quadrant regions with positive rebound and Rovsing signs. Abdominal X-ray was unremarkable. Laboratory investigations revealed leukocytosis (Table 1). Enhanced CT scan of the abdomen and pelvis revealed signs of appendicitis with a moderate amount of free fluid (Fig. 1a and b).
| Labs . | Results . | Normal range . |
|---|---|---|
| WBC | 12.5 × 10e3/uL | 4.00–11.00 |
| Hb | 12.9 g/dL | 13.5–17.2 |
| Platelets | 274 × 10e3/uL | 150–450 |
| Na | 139.0 mmol/L | 136.0–145.0 |
| K | 3.60 mmol/L | 3.60–5.00 |
| Amylase | 51.00 U/L | 25.00–125.00 |
| Lipase | 29.0 U/L | 8.0–78.0 |
| Labs . | Results . | Normal range . |
|---|---|---|
| WBC | 12.5 × 10e3/uL | 4.00–11.00 |
| Hb | 12.9 g/dL | 13.5–17.2 |
| Platelets | 274 × 10e3/uL | 150–450 |
| Na | 139.0 mmol/L | 136.0–145.0 |
| K | 3.60 mmol/L | 3.60–5.00 |
| Amylase | 51.00 U/L | 25.00–125.00 |
| Lipase | 29.0 U/L | 8.0–78.0 |
WBC = White blood cells; Hb = Haemoglobin; Na = Sodium; K = Potassium.
| Labs . | Results . | Normal range . |
|---|---|---|
| WBC | 12.5 × 10e3/uL | 4.00–11.00 |
| Hb | 12.9 g/dL | 13.5–17.2 |
| Platelets | 274 × 10e3/uL | 150–450 |
| Na | 139.0 mmol/L | 136.0–145.0 |
| K | 3.60 mmol/L | 3.60–5.00 |
| Amylase | 51.00 U/L | 25.00–125.00 |
| Lipase | 29.0 U/L | 8.0–78.0 |
| Labs . | Results . | Normal range . |
|---|---|---|
| WBC | 12.5 × 10e3/uL | 4.00–11.00 |
| Hb | 12.9 g/dL | 13.5–17.2 |
| Platelets | 274 × 10e3/uL | 150–450 |
| Na | 139.0 mmol/L | 136.0–145.0 |
| K | 3.60 mmol/L | 3.60–5.00 |
| Amylase | 51.00 U/L | 25.00–125.00 |
| Lipase | 29.0 U/L | 8.0–78.0 |
WBC = White blood cells; Hb = Haemoglobin; Na = Sodium; K = Potassium.
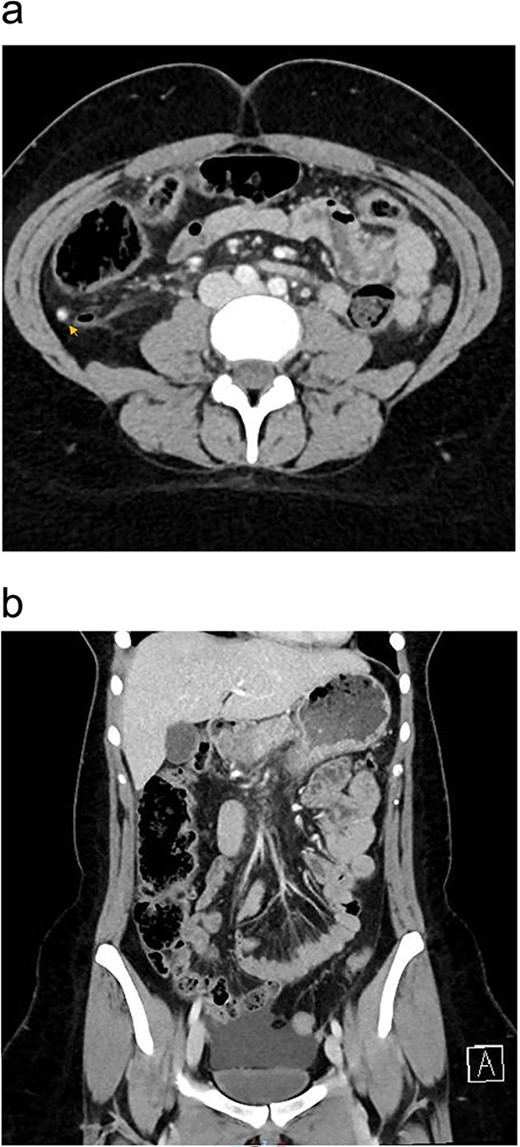
Preoperative enhanced CT abdomen and pelvis with axial (a) and coronal (b) views showing dilated appendix with hyperdense appendicolith within its lumen (arrow) associated with surrounding fat stranding and moderate amount of free fluid of −7 to 0.4 Hounsfield Units density.
The patient was taken to the operating room for a laparoscopic appendectomy. Intraoperatively, a large amount of milky fluid was discovered in the abdomen along with an inflamed appendix (Fig. 2). The fluid was aspirated and sent for bacterial culture, tuberculosis culture, cytology, and triglyceride level analysis. An attempt to identify the leak site was unsuccessful, so we proceeded with appendectomy and placed a drain in the pelvis. Postoperatively, the patient was transferred to the ward. The triglyceride level in the peritoneal fluid was markedly elevated, with a level of 2029.00 mg/dl, confirming a diagnosis of chylous ascites. All other peritoneal fluid investigations were negative.
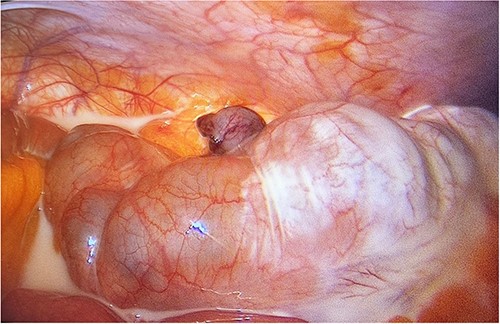
Laparoscopic view of the right lower quadrant showing chyloperitoneum with appendix tip in the middle (post ligation of appendicular artery).
The patient was managed with a low-fat diet, octreotide, and close monitoring of drain output. Over the following days, the drain output gradually diminished and became serous in color. By the third postoperative day, the drain was removed, and patient was discharged from the hospital in good condition. During follow-up in the outpatient clinic, the patient reported no active complaints and was in good health. A follow-up enhanced CT scan of the abdomen and pelvis, performed 1 month after discharge, showed no signs of recurrence (Fig. 3a and b). Histopathology results of the removed appendix confirmed the diagnosis of acute appendicitis.
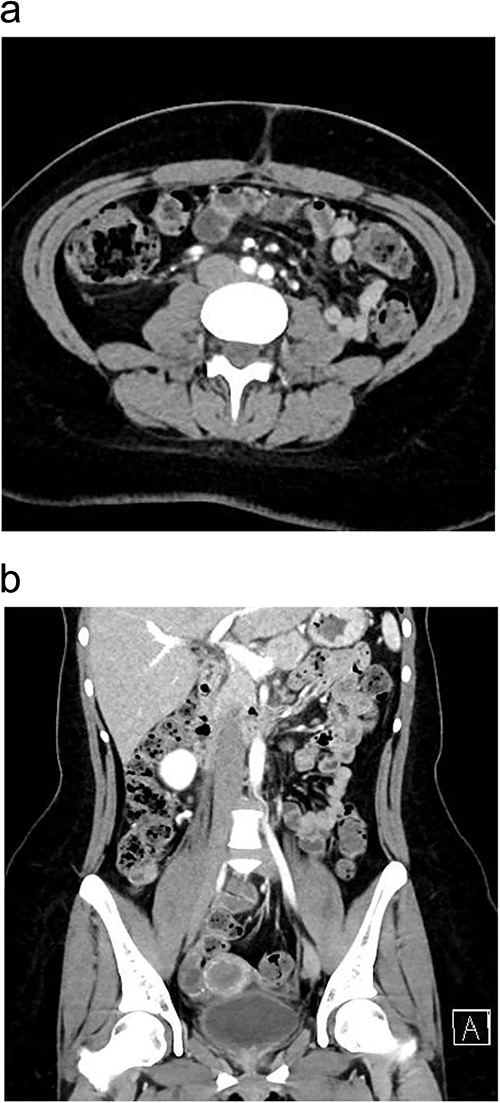
One month postoperatively enhanced CT abdomen and pelvis with axial (a) and coronal (b) views showing resolution of inflammation and no signs of recurrence of chyloperitoneum.
Discussion
Chyloperitoneum is a rare condition characterized by the presence of chyle in the peritoneal cavity. We encountered a case of acute appendicitis with accumulation of chyle in the abdomen, and the underlying cause of this incidental finding is unclear. The principal mechanisms behind chyloperitoneum as described by Aalami et al. [3] involve the exudation of chyle from dilated lymphatics on the bowel wall and within the mesentery due to lymphatics obstruction in the mesentery or cisterna chyli.
Our hypothesis behind chyloperitoneum in appendicitis is that severe inflammation may damage or obstruct chyle flow in the surrounding mesentery, leading to exudation of chyle and causing its accumulation in the peritoneal cavity (Fig. 4).
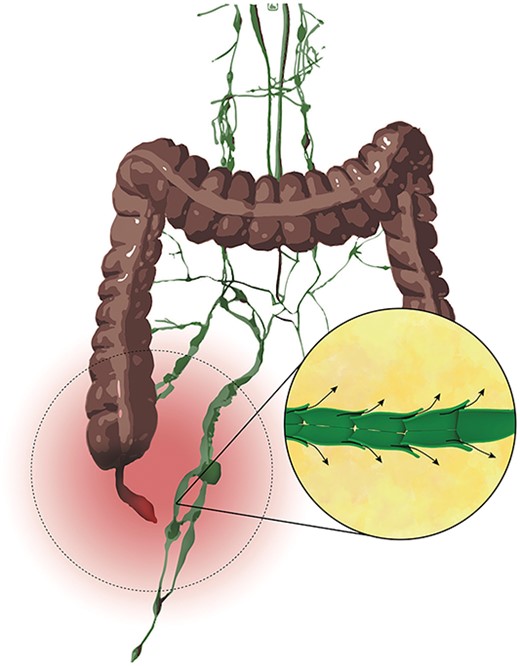
Illustritation of the hypothetical pathophysiology behind acute chyloperitoneum with appendicitis.
In reviewing the literature, similar cases were examined, analyzed, and summarized in Table 2; the mean age of patients was 33.6 years (ranging from 6 to 69 years). Males predominated with a male-to-female ratio of 3:2; majority of cases reported no history of alcohol or smoking use, and none had a history of trauma.
| Author / year . | Age . | Sex . | Smoking . | Alcohol use . | History of trauma . | Presence of appendicitis . | Other intraoperative findings . | Intervention . | Drain removal in days . | Use of TPN . | Use of octreotide . | Recurrence . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fazili et al. (1999) [5] | 25 | M | Yes | No | No | No | None | Intraperitoneal drainage | 2 | NA | NA | No recurrence in 9 months follow up |
| Fang et al. (2006) [6] | 22 | M | No | No | No | No | Lymphatic drainage from thoracic duct | Suture ligation of lymphatic leakage from thoracic duct and retroperitoneal drainage | NA | No | NA | No recurrence in 6 months follow up |
| Vettoretto et al. (2008) [7] | 69 | M | NA | NA | No | - | Small vessel hypertrophy forming little angiomas on the mesenteric side of the bowel | Intraperitoneal drainage | 7 | Yes | NA | No recurrence in 2 years follow up |
| Akbulut et al. (2010) [8] | 25 | M | No | No | No | Yes | Proximal jejunal lymphangiectasia | Appendectomy and intraperitoneal drainage | 7 | Yes | Yes | No recurrence in 1 month follow up |
| Rogdakis et al. (2011) [9] | 46 | M | Yes | Yes | No | No | None | Intraperitoneal drainage | 7 | Yes | Yes | No recurrence in 6 months follow up |
| Ozgüç et al. (2013) [10] | 32 | F | No | No | No | No | None | Intraperitoneal drainage | 7 | No | Yes | No recurrence in 9 months follow up |
| Xu et al. (2015) [11] | 6 | M | No | No | No | No | None | Prophylactic appendectomy | - | No | No | NA |
| Ul Ain et al. (2016) [12] | 32 | F | No | Yes | No | No | None | Prophylactic appendectomy and intraperitoneal drainage | ?5 | NA | NA | No recurrence in 6 months follow up |
| Kaya et al. (2017) [13] | 30 | M | NA | NA | NA | Yes | Edematous small bowel mesentery with enlarged lymphatic vessels | Appendectomy and intraperitoneal drainage | 5 | Yes | Yes | NA |
| Alamri et al. (2020) [14] | 32 | M | NA | NA | No | No | None | Intraperitoneal drainage | 7 | No | Yes | NA |
| Manco et al. (2020) [15] | 30 | F | NA | NA | No | No | None | Prophylactic appendectomy and intraperitoneal drainage | 3 | NA | NA | No recurrence in 5 months follow up |
| Apikotoa et al. (2021) [16] | 36 | F | No | No | No | No | Dilated lymphatic vessels on the surface of the small bowel | Prophylactic appendectomy and intraperitoneal drainage | 5 | No | No | No recurrence in 2 weeks follow up |
| Epelde et al. (2024) [17] | 35 | F | NA | NA | No | No | None | Intraperitoneal drainage | ?4 | No | Yes | No recurrence in 1 year follow up |
| Zenati et al. (2024) [18] | 61 | M | No | No | No | No | Dilated lymphatic vessels on the surface of the small bowel | Intraperitoneal drainage | NA | No | NA | No recurrence in 3 years follow up |
| Current study | 24 | F | No | No | No | Yes | None | Appendectomy and intraperitoneal drainage | 3 | No | Yes | No recurrence in 2 months follow up |
| Author / year . | Age . | Sex . | Smoking . | Alcohol use . | History of trauma . | Presence of appendicitis . | Other intraoperative findings . | Intervention . | Drain removal in days . | Use of TPN . | Use of octreotide . | Recurrence . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fazili et al. (1999) [5] | 25 | M | Yes | No | No | No | None | Intraperitoneal drainage | 2 | NA | NA | No recurrence in 9 months follow up |
| Fang et al. (2006) [6] | 22 | M | No | No | No | No | Lymphatic drainage from thoracic duct | Suture ligation of lymphatic leakage from thoracic duct and retroperitoneal drainage | NA | No | NA | No recurrence in 6 months follow up |
| Vettoretto et al. (2008) [7] | 69 | M | NA | NA | No | - | Small vessel hypertrophy forming little angiomas on the mesenteric side of the bowel | Intraperitoneal drainage | 7 | Yes | NA | No recurrence in 2 years follow up |
| Akbulut et al. (2010) [8] | 25 | M | No | No | No | Yes | Proximal jejunal lymphangiectasia | Appendectomy and intraperitoneal drainage | 7 | Yes | Yes | No recurrence in 1 month follow up |
| Rogdakis et al. (2011) [9] | 46 | M | Yes | Yes | No | No | None | Intraperitoneal drainage | 7 | Yes | Yes | No recurrence in 6 months follow up |
| Ozgüç et al. (2013) [10] | 32 | F | No | No | No | No | None | Intraperitoneal drainage | 7 | No | Yes | No recurrence in 9 months follow up |
| Xu et al. (2015) [11] | 6 | M | No | No | No | No | None | Prophylactic appendectomy | - | No | No | NA |
| Ul Ain et al. (2016) [12] | 32 | F | No | Yes | No | No | None | Prophylactic appendectomy and intraperitoneal drainage | ?5 | NA | NA | No recurrence in 6 months follow up |
| Kaya et al. (2017) [13] | 30 | M | NA | NA | NA | Yes | Edematous small bowel mesentery with enlarged lymphatic vessels | Appendectomy and intraperitoneal drainage | 5 | Yes | Yes | NA |
| Alamri et al. (2020) [14] | 32 | M | NA | NA | No | No | None | Intraperitoneal drainage | 7 | No | Yes | NA |
| Manco et al. (2020) [15] | 30 | F | NA | NA | No | No | None | Prophylactic appendectomy and intraperitoneal drainage | 3 | NA | NA | No recurrence in 5 months follow up |
| Apikotoa et al. (2021) [16] | 36 | F | No | No | No | No | Dilated lymphatic vessels on the surface of the small bowel | Prophylactic appendectomy and intraperitoneal drainage | 5 | No | No | No recurrence in 2 weeks follow up |
| Epelde et al. (2024) [17] | 35 | F | NA | NA | No | No | None | Intraperitoneal drainage | ?4 | No | Yes | No recurrence in 1 year follow up |
| Zenati et al. (2024) [18] | 61 | M | No | No | No | No | Dilated lymphatic vessels on the surface of the small bowel | Intraperitoneal drainage | NA | No | NA | No recurrence in 3 years follow up |
| Current study | 24 | F | No | No | No | Yes | None | Appendectomy and intraperitoneal drainage | 3 | No | Yes | No recurrence in 2 months follow up |
M = Male; F = Female; NA = Not available
| Author / year . | Age . | Sex . | Smoking . | Alcohol use . | History of trauma . | Presence of appendicitis . | Other intraoperative findings . | Intervention . | Drain removal in days . | Use of TPN . | Use of octreotide . | Recurrence . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fazili et al. (1999) [5] | 25 | M | Yes | No | No | No | None | Intraperitoneal drainage | 2 | NA | NA | No recurrence in 9 months follow up |
| Fang et al. (2006) [6] | 22 | M | No | No | No | No | Lymphatic drainage from thoracic duct | Suture ligation of lymphatic leakage from thoracic duct and retroperitoneal drainage | NA | No | NA | No recurrence in 6 months follow up |
| Vettoretto et al. (2008) [7] | 69 | M | NA | NA | No | - | Small vessel hypertrophy forming little angiomas on the mesenteric side of the bowel | Intraperitoneal drainage | 7 | Yes | NA | No recurrence in 2 years follow up |
| Akbulut et al. (2010) [8] | 25 | M | No | No | No | Yes | Proximal jejunal lymphangiectasia | Appendectomy and intraperitoneal drainage | 7 | Yes | Yes | No recurrence in 1 month follow up |
| Rogdakis et al. (2011) [9] | 46 | M | Yes | Yes | No | No | None | Intraperitoneal drainage | 7 | Yes | Yes | No recurrence in 6 months follow up |
| Ozgüç et al. (2013) [10] | 32 | F | No | No | No | No | None | Intraperitoneal drainage | 7 | No | Yes | No recurrence in 9 months follow up |
| Xu et al. (2015) [11] | 6 | M | No | No | No | No | None | Prophylactic appendectomy | - | No | No | NA |
| Ul Ain et al. (2016) [12] | 32 | F | No | Yes | No | No | None | Prophylactic appendectomy and intraperitoneal drainage | ?5 | NA | NA | No recurrence in 6 months follow up |
| Kaya et al. (2017) [13] | 30 | M | NA | NA | NA | Yes | Edematous small bowel mesentery with enlarged lymphatic vessels | Appendectomy and intraperitoneal drainage | 5 | Yes | Yes | NA |
| Alamri et al. (2020) [14] | 32 | M | NA | NA | No | No | None | Intraperitoneal drainage | 7 | No | Yes | NA |
| Manco et al. (2020) [15] | 30 | F | NA | NA | No | No | None | Prophylactic appendectomy and intraperitoneal drainage | 3 | NA | NA | No recurrence in 5 months follow up |
| Apikotoa et al. (2021) [16] | 36 | F | No | No | No | No | Dilated lymphatic vessels on the surface of the small bowel | Prophylactic appendectomy and intraperitoneal drainage | 5 | No | No | No recurrence in 2 weeks follow up |
| Epelde et al. (2024) [17] | 35 | F | NA | NA | No | No | None | Intraperitoneal drainage | ?4 | No | Yes | No recurrence in 1 year follow up |
| Zenati et al. (2024) [18] | 61 | M | No | No | No | No | Dilated lymphatic vessels on the surface of the small bowel | Intraperitoneal drainage | NA | No | NA | No recurrence in 3 years follow up |
| Current study | 24 | F | No | No | No | Yes | None | Appendectomy and intraperitoneal drainage | 3 | No | Yes | No recurrence in 2 months follow up |
| Author / year . | Age . | Sex . | Smoking . | Alcohol use . | History of trauma . | Presence of appendicitis . | Other intraoperative findings . | Intervention . | Drain removal in days . | Use of TPN . | Use of octreotide . | Recurrence . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fazili et al. (1999) [5] | 25 | M | Yes | No | No | No | None | Intraperitoneal drainage | 2 | NA | NA | No recurrence in 9 months follow up |
| Fang et al. (2006) [6] | 22 | M | No | No | No | No | Lymphatic drainage from thoracic duct | Suture ligation of lymphatic leakage from thoracic duct and retroperitoneal drainage | NA | No | NA | No recurrence in 6 months follow up |
| Vettoretto et al. (2008) [7] | 69 | M | NA | NA | No | - | Small vessel hypertrophy forming little angiomas on the mesenteric side of the bowel | Intraperitoneal drainage | 7 | Yes | NA | No recurrence in 2 years follow up |
| Akbulut et al. (2010) [8] | 25 | M | No | No | No | Yes | Proximal jejunal lymphangiectasia | Appendectomy and intraperitoneal drainage | 7 | Yes | Yes | No recurrence in 1 month follow up |
| Rogdakis et al. (2011) [9] | 46 | M | Yes | Yes | No | No | None | Intraperitoneal drainage | 7 | Yes | Yes | No recurrence in 6 months follow up |
| Ozgüç et al. (2013) [10] | 32 | F | No | No | No | No | None | Intraperitoneal drainage | 7 | No | Yes | No recurrence in 9 months follow up |
| Xu et al. (2015) [11] | 6 | M | No | No | No | No | None | Prophylactic appendectomy | - | No | No | NA |
| Ul Ain et al. (2016) [12] | 32 | F | No | Yes | No | No | None | Prophylactic appendectomy and intraperitoneal drainage | ?5 | NA | NA | No recurrence in 6 months follow up |
| Kaya et al. (2017) [13] | 30 | M | NA | NA | NA | Yes | Edematous small bowel mesentery with enlarged lymphatic vessels | Appendectomy and intraperitoneal drainage | 5 | Yes | Yes | NA |
| Alamri et al. (2020) [14] | 32 | M | NA | NA | No | No | None | Intraperitoneal drainage | 7 | No | Yes | NA |
| Manco et al. (2020) [15] | 30 | F | NA | NA | No | No | None | Prophylactic appendectomy and intraperitoneal drainage | 3 | NA | NA | No recurrence in 5 months follow up |
| Apikotoa et al. (2021) [16] | 36 | F | No | No | No | No | Dilated lymphatic vessels on the surface of the small bowel | Prophylactic appendectomy and intraperitoneal drainage | 5 | No | No | No recurrence in 2 weeks follow up |
| Epelde et al. (2024) [17] | 35 | F | NA | NA | No | No | None | Intraperitoneal drainage | ?4 | No | Yes | No recurrence in 1 year follow up |
| Zenati et al. (2024) [18] | 61 | M | No | No | No | No | Dilated lymphatic vessels on the surface of the small bowel | Intraperitoneal drainage | NA | No | NA | No recurrence in 3 years follow up |
| Current study | 24 | F | No | No | No | Yes | None | Appendectomy and intraperitoneal drainage | 3 | No | Yes | No recurrence in 2 months follow up |
M = Male; F = Female; NA = Not available
Most patients had no prior surgeries except for three. One of whom had an appendectomy, while the remaining two had non-abdominal surgeries. Acute right iliac fossa pain was a common symptom in all cases, but only 3 out of 15 had operative findings of appendicitis (20%), with the remaining cases lacked other identifiable pathologies to explain the symptoms.
While retroperitoneal exploration was carried out in 3 out of 15 cases, only 1 patient showed lymphatic leakage from thoracic duct and was repaired through suture ligation [6]; all other cases were unable to locate a leak site.
Drainage played a significant role in management, either alone (53.3%) or in conjunction with appendectomy (46.7%). Fortunately, all cases showed no recurrence of chyloperitoneum during follow-up.
In our case and similar cases in the literature, chyle leakage was self-limiting, supporting the theory that it is linked to mesenteric inflammation, and resolution of the inflammation led to a decrease in the rate of chyle leakage and eventual cessation.
Based on our review of the literature and our own experience, we suggest adopting a conservative approach when managing acute chyloperitoneum associated with appendicitis. Avoiding aggressive interventions is key to minimizing complications (Fig. 5).
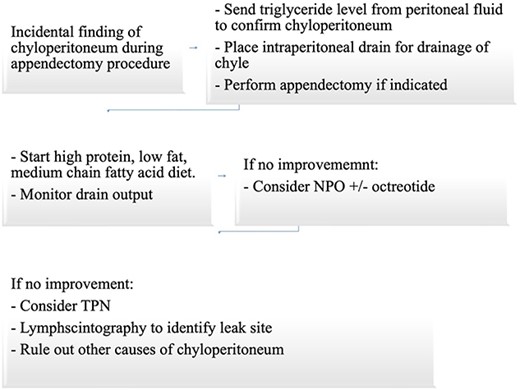
Suggested approach for incidental finding of chyloperitoneum intraoperatively during appendectomy.
Intraperitoneal drainage, along with appendectomy if indicated, accompanied by observation is sufficient as demonstrated by our findings and similar cases in the literature, unless a specific leakage site or another underlying pathology is confirmed.
Conclusion
Although there is no established relationship between appendicitis and chyloperitoneum, we hypothesize that surrounding mesenteric inflammation may be a connecting factor.
Given the lack of a standardized treatment approach in the literature for incidentally discovered acute chyloperitoneum during an appendectomy, a conservative management approach is advised in such cases. This approach includes intraperitoneal drainage, appendectomy if needed, and careful observation. Our proposed management strategy aligns with findings from the literature review and supports conservative management as a safe and effective treatment modality for this rare condition.
Further studies are needed to elucidate how appendicitis could be related to chyloperitoneum, to guide diagnostic and therapeutic strategies to effectively manage this condition.
Acknowledgements
A special thanks to Naser F. Ashour for his illustration of the hypothetical pathophysiology.
Conflict of interest statement
None declared.
Funding
None declared.



