-
PDF
- Split View
-
Views
-
Cite
Cite
Dale Mortenson, Anna Perez, Biliary anatomic variant and recurrent acute cholecystitis, cholelithiasis in gallbladder remnant in patient with autosomal dominant polycystic kidney disease, Journal of Surgical Case Reports, Volume 2024, Issue 7, July 2024, rjae467, https://doi.org/10.1093/jscr/rjae467
Close - Share Icon Share
Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited renal disorder. ADPKD is not only associated with progression of renal disease, but also several hepatobiliary manifestations. This report is of a 49-year-old female with recurrent cholelithiasis and cholecystitis following subtotal cholecystectomy in the context of aberrant biliary anatomy and ADPKD. There were significant adhesions obscuring the cystic duct, necessitating the second cholecystectomy be performed open. The right posterior hepatic duct was adhered to the gallbladder wall and was perforated while attempting to remove the gallbladder remnant. The duct was repaired over a T-tube, without any subsequent biliary leak. The cystic duct was hugely dilated and impacted with stones down to the junction with the common bile duct, which were evacuated, and the cystic duct was oversewn along with the remnant of the gallbladder wall. The recovery course was unremarkable.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is a progressive condition characterized by numerous kidney cysts bilaterally and is the most common inherited renal disorder. Patients with polycystic kidney disease frequently present with several extrarenal manifestations including polycystic liver, common bile duct dilatation and other hepatobiliary morphologic abnormalities [1, 2]. Polycystic liver disease has been attributed to failure of embryonic biliary ducts to regress resulting in cystic malformations of bile ducts in the peripheral biliary tree [3]. Distortions in biliary architecture have been noted in polycystic liver disease. These distortions are considered to be caused by mass effect of the hepatic cysts [4]. Variant anatomy of the bile ducts has been identified as a major factor in bile duct injury in cholecystectomy [5]. This case is an example of biliary duct malformations in a patient with ADPKD, resulting in adherence of a right posterior hepatic duct to the gallbladder and bile leak during completion cholecystectomy.
Case presentation
A 49-year-old female with a history of ADPKD and multiple liver cysts, 13-months status post subtotal cholecystectomy, endoscopic retrograde cholangiopancreatography with biliary sphincterotomy and balloon stone extraction, presented to the emergency department with fever and a 4-day history of discomfort in the right upper quadrant. At the time of the initial cholecystectomy, total cholecystectomy could not be performed due to extensive adhesions, and subtotal cholecystectomy with complete evacuation of the stones was done instead. Magnetic resonance cholangiopancreatography (MRCP) done at that time showed a dilated common bile duct (CBD) to 1.5 cm, intra and extrahepatic biliary dilatation, multiple liver cysts measuring up to 2 cm, and multiple stones in the CBD, including at the level of the ampulla (Fig. 1). Computerized tomography scan at the time of the second admission showed acute cholecystitis and she had mildly elevated transaminases, but there was no evidence of cholestasis or pancreatitis. MRCP showed minor proximal intrahepatic biliary dilation and cholelithiasis but no duct calculi (Figs 2–4). A laparoscopic cholecystectomy was attempted with extensive adhesiolysis between the gallbladder and surrounding omentum and mesocolon, but the cystic duct could not be isolated, and ultimately the cased was converted to open. While removing the gallbladder in a top-down fashion, a small bile leak was identified right at the closed end of the gallbladder remnant, where the bile duct was adhered to the gallbladder wall. Intraoperative cholangiogram was obtained through a small perforation in a right posterior bile duct at the site of attachment, which showed right posterior, right anterior and left hepatic ducts of equal size converged at a trifurcation (Fig. 5). The duct was repaired over a T-tube and observed throughout the rest of the case and no bile leak was identified. The gallbladder was opened along its lateral edge, revealing a hugely dilated cystic duct with ~10 to 12 marble-sized stones packed within the gallbladder remnant and cystic duct down to the juncture with the common bile duct. All the stones were removed and the safe portion of the gallbladder remnant was resected leaving the side attached to the bile duct in situ due to the Mirizzi anatomical variant. The cystic duct was oversewn along with the remnant of the gallbladder wall. Pathology showed acute-on-chronic cholecystitis with cholelithiasis. Repeat MRCP showed no retained stones in the biliary tree. The recovery course was unremarkable.
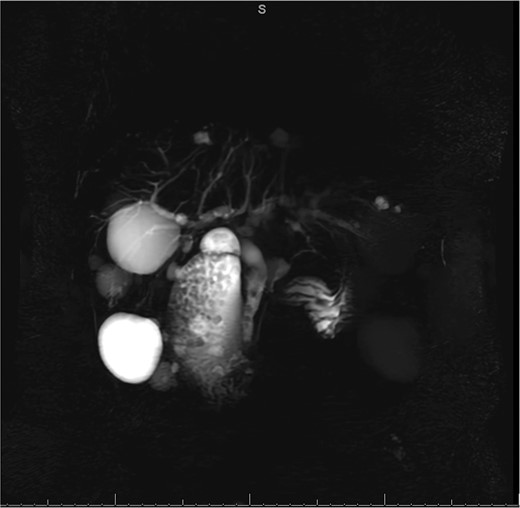
MRCP from initial hospitalization, including cysts in the area of the gallbladder fossa, cholelithiasis, choledocholithiasis and CBD dilation, measured to be 1.5 cm (measurement not included in image).
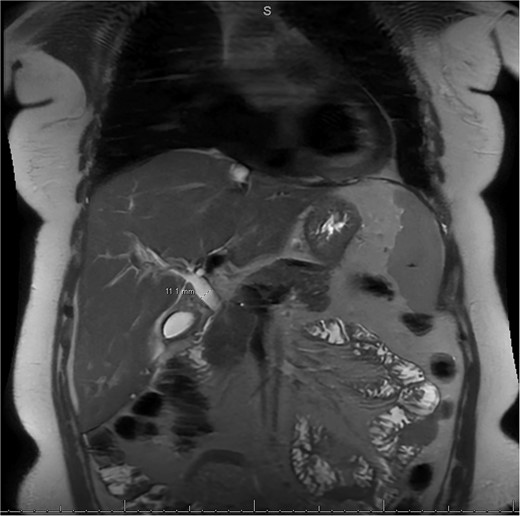
MRI/MRCP from second hospitalization demonstrating CBD of 1.1 cm.
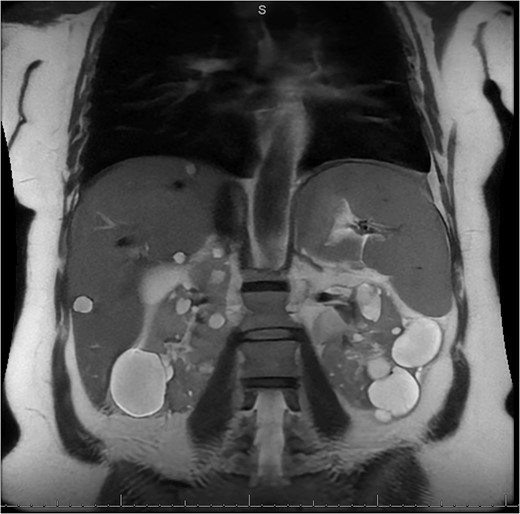
MRI/MRCP from second hospitalization demonstrating liver and kidney cysts.
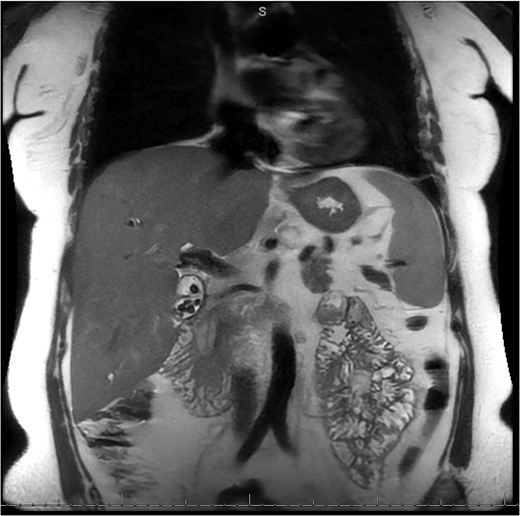
MRI/MRCP from second hospitalization demonstrating recurrent cholelithiasis.
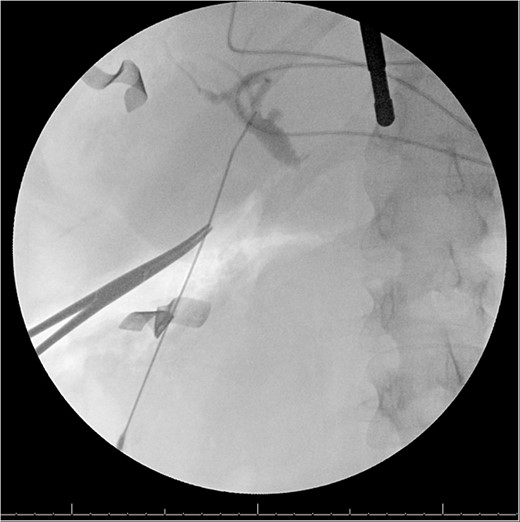
Intraoperative cholangiogram from second procedure demonstrating trifurcation of the right posterior, right anterior and left hepatic ducts, as well as a dilated cystic duct.
Discussion
ADPKD is the most common inherited renal pathology and is associated with myriad extrarenal manifestations. Up to 80% of patients with ADPKD present with liver cysts which result from failed regression and eventual malformation of peripheral bile ducts, which may further distort the morphology of the surrounding liver parenchyma and bile duct anatomy during development [3, 6]. While a triple confluence of the right anterior, right posterior and left hepatic ducts is relatively common, adherence of the right posterior hepatic duct to the gallbladder resulting in bile duct injury during cholecystectomy has not previously been described [5]. Adhesions and fibrosis in the area of the cystic duct has previously been identified as a possible harbinger of impacted stones [7]. Extensive adhesions in the area of the gallbladder were initially noted during the first cholecystectomy, thereby excluding surgery as the sole cause of the adhesions. Increased risk of recurrent cholelithiasis has been noted in patients with subtotal cholecystectomy when compared with those who only remained with a long cystic duct stump, which has been attributed to residual gallbladder mucosa [8]. Biliary tract disease, not limited to common bile duct dilatation, has been identified as an independent extrarenal manifestation of ADPKD [9]. Variant biliary anatomy has been previously associated with bile duct injury during cholecystectomy [5, 10].
In this case, the proximity of the right posterior hepatic duct with the gallbladder in addition to the extensive adhesions makes it difficult to determine if the fusion between the bile duct and the gallbladder was congenital or developed due to biliary disease in the context of ADPKD. Given the increased association of variant biliary anatomy with ADPKD and liver cysts, this population may be at increased risk of bile duct injury during cholecystectomy.
Conflict of interest statement
The authors have no conflict of interest.
Funding
The authors received no funding for the writing of this case report.
Consent
Consent was obtained from the participant in this study.



