-
PDF
- Split View
-
Views
-
Cite
Cite
Andrew Tse, Natalie Phan, Salah Ayoubi, Douglas Fenton-Lee, Necrotic mega-stomach from superior mesenteric artery syndrome, Journal of Surgical Case Reports, Volume 2024, Issue 7, July 2024, rjae463, https://doi.org/10.1093/jscr/rjae463
Close - Share Icon Share
Abstract
Superior mesenteric artery (SMA) syndrome is a rare cause of proximal bowel obstruction due to duodenal compression by the SMA. The morbidity and mortality associated with delayed diagnosis and its complications make it an important differential cause for bowel obstruction. We report a case of mega-stomach secondary to SMA syndrome requiring total gastrectomy. An 18-year-old male presented with vomiting, abdominal pain and shock after a buffet. Computed tomography (CT) imaging revealed a grossly distended stomach (113 × 187 × 350mm) and a transition point at the third part of the duodenum, along with pneumatosis and portal venous gas. Emergency gastroscopy showed blood and necrotic mucosa. Laparotomy confirmed full thickness necrosis and the patient underwent a total gastrectomy with Roux-en-Y reconstruction. Postoperatively, he had a brief intensive care stay and recovered without complications. This case underscores the importance of considering SMA syndrome during presentations of acute gastric dilatation.
Introduction
Superior mesenteric artery (SMA) syndrome, also known as Wilkie’s or Cast’s syndrome, involves compression of the duodenum between the SMA and abdominal aorta (AA) due to loss of fatty tissue. This rare condition has an unknown incident but thought to range between 0.013% and 0.3% [1]. Reports of associated acute gastric dilatation with ischaemia, pneumatosis, or portal venous gas are infrequent and can be fatal [2–7]. Gastrectomy cases are mainly reported in East Asian countries [8, 9]. We present an Australian case of severe acute gastric dilatation secondary to SMA syndrome with patient consent, leading to gastric necrosis and requiring total gastrectomy.
Case report
An 18-year-old male presented with severe abdominal pain, distension, and vomiting after a buffet meal. He had a BMI of 22 (weight 71 kg, height 181 cm) and no significant medical history or recent weight loss. He was normotensive (110/80), tachycardic (>120 bpm), and febrile (38.9°C). Examination revealed lethargy, a grossly tympanic, distended and tender abdomen. Biochemical tests showed severe lactic acidosis (pH 7.13, lactate 13.7), leukocytosis (white cell count 21×108) and acute kidney injury (creatinine 203 μmol/L, glomerular filtration rate 40 ml/mn/1.73m2). CT scan revealed acute gastric dilatation (35×19×12 cm), gastric pneumotosis and portal venous gas (Figs 1 and 2) with aortomesenteric angle of 5° and distance of 6 mm (Fig. 3). There were broad differentials considered at the time of presentation including both mechanical and functional aetiologies (Table 1).
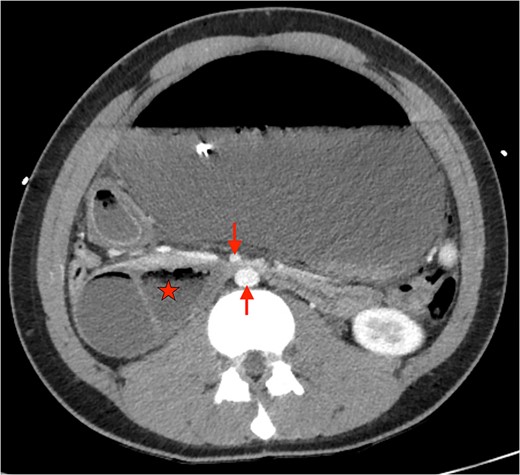
Axial CT showing severely distended stomach and dilated duodenum with transition point between SMA and aorta. (★: Duodenum. ↓: Superior mesenteric artery. ↑: abdominal aorta).
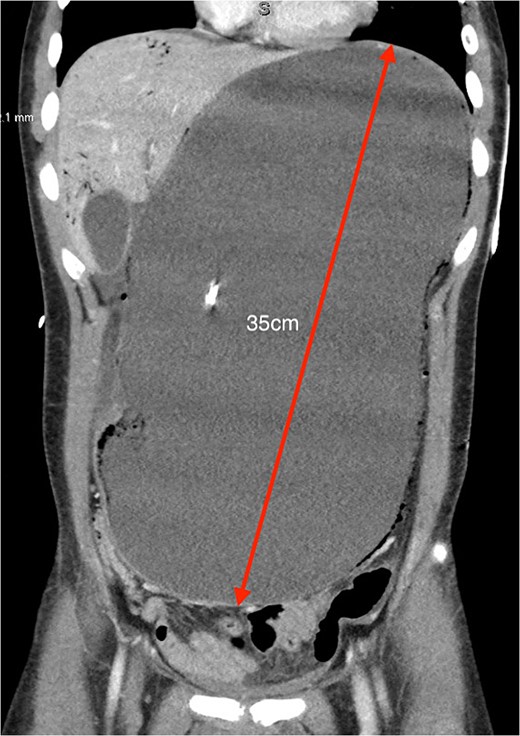
Coronal CT showing severely distended stomach to the pelvis (35 cm in greatest length) with evidence of pneumotosis and portal venous gas.
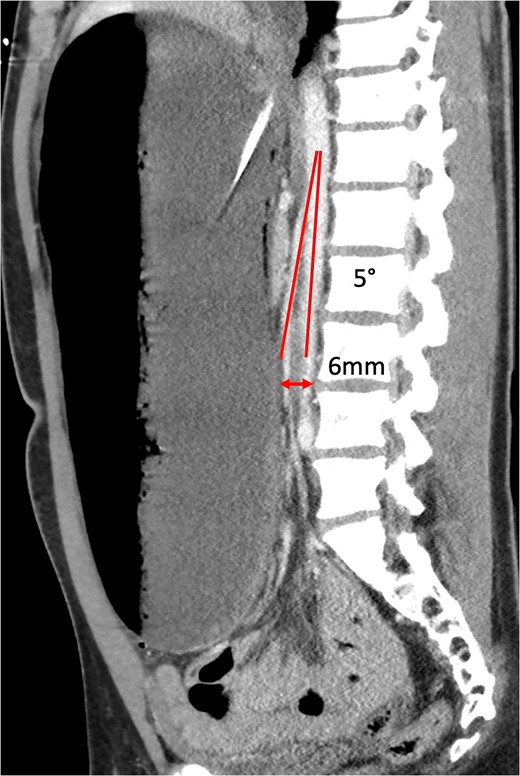
Sagittal CT showing aortomesenteric angle (5°) and aortomesenteric distance (6 mm).
Differential diagnoses for gastric outlet obstruction and acute gastric dilatation
| Superior mesenteric artery syndrome |
| Severe gastritis / Peptic ulcer disease |
| Gastroparesis |
| Pancreatitis |
| Gastric volvulus |
| Neoplasm |
| Crohn’s disease |
| Annular pancreas |
| Bezoar |
| Bouveret syndrome |
| Intramural haematoma |
| Gastric tuberculosis |
| Superior mesenteric artery syndrome |
| Severe gastritis / Peptic ulcer disease |
| Gastroparesis |
| Pancreatitis |
| Gastric volvulus |
| Neoplasm |
| Crohn’s disease |
| Annular pancreas |
| Bezoar |
| Bouveret syndrome |
| Intramural haematoma |
| Gastric tuberculosis |
Differential diagnoses for gastric outlet obstruction and acute gastric dilatation
| Superior mesenteric artery syndrome |
| Severe gastritis / Peptic ulcer disease |
| Gastroparesis |
| Pancreatitis |
| Gastric volvulus |
| Neoplasm |
| Crohn’s disease |
| Annular pancreas |
| Bezoar |
| Bouveret syndrome |
| Intramural haematoma |
| Gastric tuberculosis |
| Superior mesenteric artery syndrome |
| Severe gastritis / Peptic ulcer disease |
| Gastroparesis |
| Pancreatitis |
| Gastric volvulus |
| Neoplasm |
| Crohn’s disease |
| Annular pancreas |
| Bezoar |
| Bouveret syndrome |
| Intramural haematoma |
| Gastric tuberculosis |
Nasogastric decompression drained 6.5 L of coffee ground and bilious fluid. Intravenous broad-spectrum antibiotics and high-dose proton inhibitors were initiated. He was responsive to initial crystalloid resuscitation and reported symptom improvement. The patient was then transferred to intensive care unit (ICU) for continual monitoring and further fluid resuscitation. Within a few hours, he developed worsening abdominal pain, peritonism, and tachycardia (>140 bpm).
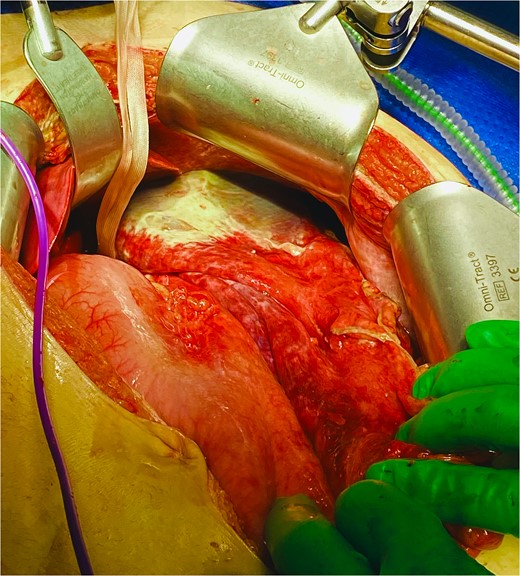
He was expedited to theatre, commencing with gastroscopy to exclude an intraluminal mechanical obstruction. There was widespread mucosal ulceration and necrosis (Figs 4–7) necessitating a laparotomy and assessment for transmural necrosis. The stomach was globally ischaemic with gangrene at the fundus (Figs 8–10) and hence a total gastrectomy was performed and reconstructed with roux-en-y oesophagojejunostomy and jejunojejunostomy.
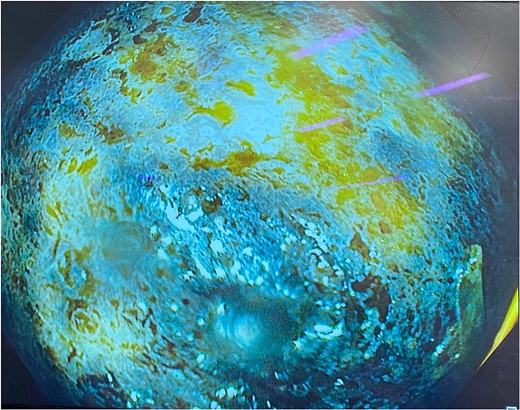
Gastroscopy showing multiple areas of mucosal ulceration and ischaemia.
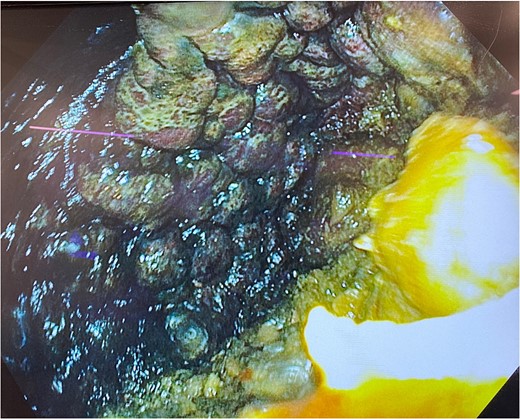
Gastroscopy showing multiple areas of mucosal ulceration and ischaemia.
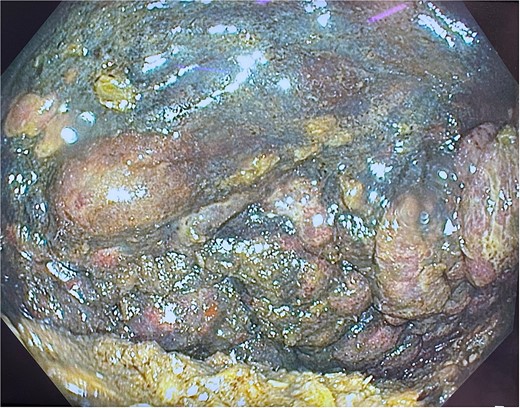
Gastroscopy showing multiple areas of mucosal ulceration and ischaemia.
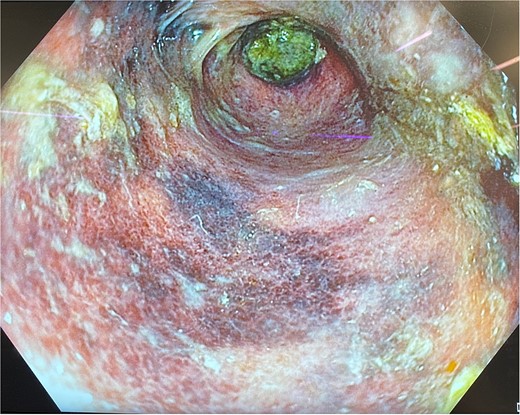
Gastroscopy showing multiple areas of mucosal ulceration and ischaemia.
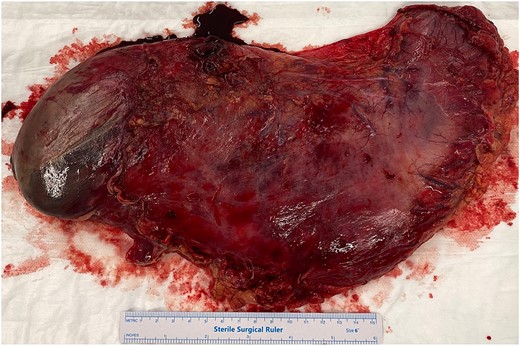
Total gastrectomy specimen showing areas of gangrene and necrosis.
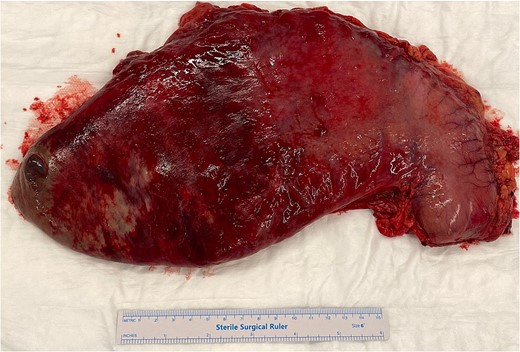
Total gastrectomy specimen showing areas of gangrene and necrosis.
Total gastrectomy specimen showing areas of gangrene and necrosis.
Postoperatively, the patient spent 5 days in ICU and 8 days in the ward. He experienced transient ischaemic hepatitis with transaminases peaking on postoperative Day 3 (alanine transaminase 1460 μ/L, aspartate aminotransferase 1260 μ/L), followed by rapid recovery thereafter. After 5 days of total parental nutrition and nil by mouth, a barium swallow confirmed no anastomotic leaks or strictures. The patient progressed to pureed diet and had an uneventful recovery. At follow-up, he had returned to normal diet and daily activities.
Histopathology confirmed widespread ischaemic changes marked oedema, blood, and extensive mucosal necrosis with multiple focal areas of full thickness necrosis.
Discussion
SMA syndrome is a defined disorder where gastrointestinal obstruction occurs due to compression of the third part of the duodenum between SMA and the AA. It was first described by Von Rokitansky in 1842 [10] and later further reported on by Wilkie [11]. It often presents with non-specific abdominal signs and symptoms and frequently is a diagnosis of exclusion [1].
SMA syndrome is more prevalent in females and people aged between 18 and 35 years, following rapid growth that exceeds compensatory weight gain. Patient’s with SMA syndrome characteristically have associated severe weight loss, where causes include but are not limited to: catabolic conditions, eating disorders, malabsorption, post-surgery weight loss, cerebral palsy, acquired immunodeficiency syndrome, corrective spinal surgery, or compression (e.g. hip spica or body casts) [1, 12]. Anatomically, a high fixation of the ligament of Treitz or low origin of the SMA, as well as exaggerated lumbar lordosis, can also cause upward displacement of the duodenum and narrow the aortomesenteric angle [13]. Aside from being a young adult, our patient did not have other typical characteristics of SMA syndrome.
The normal aorto-mesenteric angle is between 38° and 65° [14] and distance is 10–28 mm [15], whereby fibrofatty tissue bolsters the space between SMA and AA. An angle ≤22° and distance ≤8 mm is highly sensitive and specific as a radiological diagnostic criteria for SMA syndrome [16]. Our patient’s respective measurements of 5° and 6 mm fall far below the defined thresholds.
Similar to our patient, several case reports have described an onset of acute gastric dilatation and SMA syndrome following buffet meals or binge eating [4, 6, 8, 17, 18]. As Sakurai et al. suggested, an excessive eating episode has likely caused mega stomach secondary to SMA syndrome, but it is unclear whether SMA syndrome was the cause or result of acute gastric dilatation [17]. Our hypothesis is that following acute dilatation of the gastric body, the stomach applies pressure on the third part of the duodenum as well as SMA and AA. In addition, the angle between the oesophagus and the right crus of the hiatus becomes more acute and the oesophagogastric junction acts as a one way valve [4]. Consequently, a closed loop obstruction occurs raising intragastric pressure and resulting in vascular insufficiency. The pressure within the dilated stomach must surpass 20 cm H2O and exceed the gastric venous pressure in order to induce mucosal ischemia. The decline in gastric circulation compromises the integrity of the gastric wall, culminating in severe complications such as dehydration, metabolic alkalosis, and mucosal necrosis. Furthermore, acute gastric dilation elevates intra-abdominal pressure, leading to systemic circulatory failure due to the collapse of the inferior vena cava [6].
Our patient underwent total gastrectomy which was consistent with other reports describing subtotal or total gastrectomy in operative management of gastric necrosis in SMA syndrome [8, 9]. One case report described primary suturing with an omental patch to close the perforation and performed duodenal decompression [19]. In the setting of viable gastric tissue laparoscopic duojejunostomy has also been described [13, 20]. To our knowledge, this case report is the first in Australia to describe acute gastric dilatation with necrosis, secondary to SMA syndrome and requiring total gastrectomy.
Conclusion
SMA syndrome is a rare and challenging diagnosis. Acute gastric dilatation and necrosis secondary to SMA syndrome is even rarer. Further research is needed to understand whether SMA syndrome is a cause or result of acute gastric dilatation following binge eating.
Author contributions
Tse collated data and prepared the manuscript. Phan and Ayoubi edited the manuscript. Fenton-Lee conceived the study idea and design.
Conflict of interest statement
None declared.
Funding
No funding was received.
Data availability
Available upon request with corresponding author.



