-
PDF
- Split View
-
Views
-
Cite
Cite
Miguel Serpa-Irizarry, Marilee Tiru-Vega, Carolina Vazquez-Rivera, Brandon Torres-Rivera, Keila Rivera-Roman, Segundo Rodriguez-Quilichini, A rare presentation of a rare entity: giant condyloma (Buschke–Löwenstein) tumor, Journal of Surgical Case Reports, Volume 2024, Issue 7, July 2024, rjae459, https://doi.org/10.1093/jscr/rjae459
Close - Share Icon Share
Abstract
Giant condyloma accuminata or Buschke–Lowenstein tumor is a rare entity characterized by a large verrucous or cauliflower-shaped lesion primarily affecting the anogenital region. It forms part of a disease spectrum between classic condyloma accuminata and squamous cell carcinoma. Classically, it is thought to arise from previous human papilloma virus infection. Surgical management is usually the treatment of choice despite their high rate of soft tissue infiltration and recurrence. We herein describe a case of a 40-year-old male patient with cystic fibrosis diagnosed with giant condyloma accuminata without human papilloma virus or other paradigmatic risk factors that was treated with near-total surgical resection.
Introduction
Giant condyloma accuminata (GCA) or Buschke–Lowenstein (BL) tumor is a rare entity with the sum of total cases reported approximating 200 cases [1]. GCA is characterized by a large verrucous or cauliflower-shaped lesion primarily affecting the anogenital regions. Histologically, these lesions demonstrate increased mitotic activity, papillomatosis, acanthosis and tendency to infiltrate adjacent tissues [2]. It forms part of a disease spectrum between classic condyloma accuminata and squamous cell carcinoma (SCC), since in many cases, these tumors harbor areas of local invasion [3].
Available literature is scarce and consists mostly of case reports or case series. First described in 1925, its prevalence is still unknown, although some authors have estimated it to be 0.01% among sexually active individuals [4, 5]. Most of the available data suggest GCA to arise as a sexually transmitted disease secondary to human papilloma virus (HPV) infection. Additionally, vertical transmission from mother to child has also been reported. HPV variants 6 and 11 tend to be the most commonly associated with this pathology, although other subtypes have also been reported. We herein describe a case of a 40-year-old male patient with cystic fibrosis and HPV-negative serology without typical risk factors that presented with GCA and was treated with conservative near total surgical resection.
Case presentation
A 40-year-old heterosexual male with a medical history of cystic fibrosis presented to the colorectal surgery clinics due to severe rectal pain from a large perianal mass of 2 years of evolution. Initial symptoms included anorectal pruritus and discomfort with bowel movements. Primary care provider recommended topical ointments and aggressive hygiene to no avail. Due to increased size and discomfort, activities of daily living including walking, sitting and defecating became progressively challenging and disabling. Associated symptoms included unintentional 20 lb weight loss during those 2 years and bright-red bloody stools. He denied fatigue, fever, chills, yellowing of skin, previous history of warts, anogenital infections, oral ulcers, skin rashes, arthralgias, myalgias, abdominal pain or diarrhea. He further denied sexual intercourse since 4 years prior to the appearance of the lesion, as well as, toxic habit history including alcohol, smoking or illicit drug use.
Physical examination revealed an exophytic vegetative lesion, occupying the entire perianal region and protruding beyond the glutes (Fig. 1). The lesion measured ~9 × 7 cm minimal involvement of the anal canal. There were no associated perianal fistulas identified, and sphincter tone was preserved on rectal examination. Serologic testing for HIV, hepatitis B (HBsAg), hepatitis C (anti-HCV ELISA), syphilis (VDRL) and human papillomavirus (HPV) were all negative.
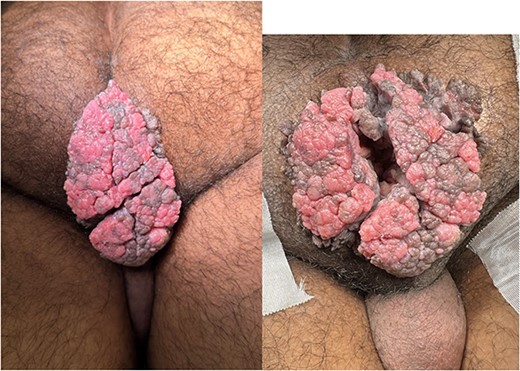
Posterior view of the anogenital region with an exophytic vegetative lesion measuring ~9 × 7 cm.
Due to the concern for peri-rectal malignancy in addition to its distressing presentation, the possibility of diagnostic and therapeutic surgical intervention was entertained. The patient was taken to the operating room and, under spinal anesthesia, placed in a jack-knife position. Rectal examination ruled out invasive components. A circumferential, near-total excision was performed, carefully dissecting the tumor off from the sphincter complex. The wound was left open to heal by secondary intention. The pathology revealed multiple foci of high-grade squamous intraepithelial lesion (HSIL/AIN3) arising in a GCA (Fig. 2). HPV testing with immunohistochemistry for P16 was not overexpressed. The patient was discharged with close follow-up and no immediate post-procedural complications.
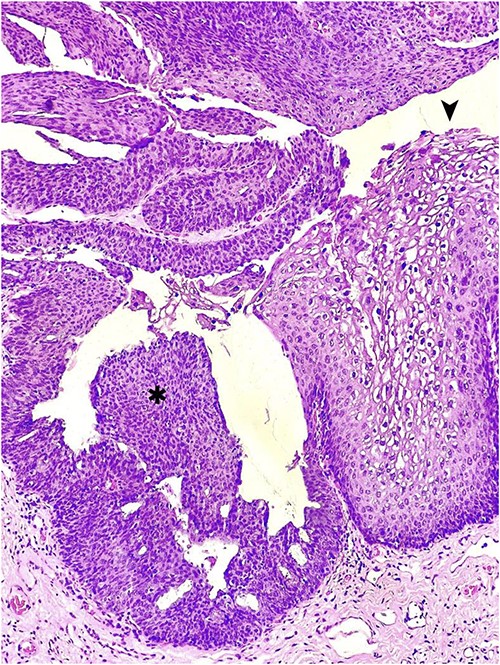
Hematoxylin and eosin staining of an anorectal GCA, also known as BLT; a hyperplastic papillary exophytic squamous epithelium is appreciated, with prominent fibrovascular cores and koilocytosis (➤) that is confined to the upper third of squamous epithelium on the left.
Discussion
GCA is classically an anogenital tumor characterized by a benign histology and a tendency for destructive growth, local invasion and malignant potential [6]. Tissue sampling, along with history and physical examination, is required for definite diagnosis. HPV infection is thought to be the causative factor, with low-risk variants 6 and 11 being the most common. Immunocompromised individuals, such as those on immunosuppressive drugs, HIV-positive status, or with history of solid organ transplant are more likely to develop the disease or suffer from rapid progression [7]. With a typical male predominance, other risk factors include multiple sexual partners, ano-receptive intercourse, chronic genital infections, smoking and poor-hygiene [2, 6, 8]. The main differential diagnosis for GCA is a verrucous carcinoma, which is a type of well-differentiated SCC [9]. Although previously thought to be part of the same disease, recent studies suggest that they are separate entities [7]. Our case is worth highlighting since our patient presented without history of HPV or genital warts and lacked all of the other classic risk factors aside from male sex. Additionally, even though cystic fibrosis is known to have extrapulmonary complications that include the gastrointestinal tract, condylomas are not considered to be one of them [10]. With regards to the final pathology, P16 immunohistochemistry, which is used as a surrogate for oncogenic HPV subtypes, was not overexpressed and although tissue evaluation ruled out verrucous carcinoma, it did show evidence of high-grade dysplasia, which is uncommon for GCA [7].
The management of this disease parallels its understudied presentation and ranges from conservative management to systemic therapy and surgical resection, with radical excision considered the treatment of choice [2]. However, due to the size, area, and invasive nature of these lesions, complete resection is often difficult and carries high risk of morbidity. Furthermore, the recurrence rate has been described to be as high as more than 50% even after radical resection [6]. Other treatment modalities used alone or in combination with surgery have been described with inferior results, including topical agents such as 5-fluorouracil and podophyllin as well as systemic chemotherapy, immunotherapy and radiation therapy [2, 7, 9, 11]. Although previously thought to be higher, recent studies suggest that mortality from this disease is low and usually related to infectious complications [1].
In our case, the patient decided to undergo a conservative surgery focused on symptom reduction and avoidance of high morbidity procedures. Therefore, we proceeded with a near total excision, preserving the sphincter complex and avoiding complex flaps or reconstructions. The wound was left open to heal by secondary intention. No infections developed and fecal continence was preserved. One of the disadvantages to this conservative approach is the possibility of a remaining lesion and malignant transformation. For this reason, patient remains with short-interval follow-up and regular anoscopic examinations.
In conclusion, GCA remains a rare entity with variable presentations and without specific treatment guidelines. Thus, the surgical management of this disease may be individualized and tailored to account for its multiple presentations in a patient-centered approach.
Conflict of interest statement
None declared.
Funding
None declared.



