-
PDF
- Split View
-
Views
-
Cite
Cite
Yoshihiro Sato, Shingo Tsujinaka, Tomoya Miura, Yoh Kitamura, Kentaro Sawada, Atsushi Mitamura, Kuniharu Yamamoto, Toru Nakano, Yu Katayose, Chikashi Shibata, Mesorectal thromboembolism with increased 18F-fluorodeoxyglucose uptake during positron emission tomography/computed tomography in a patient with non-small cell lung cancer, Journal of Surgical Case Reports, Volume 2024, Issue 7, July 2024, rjae457, https://doi.org/10.1093/jscr/rjae457
Close - Share Icon Share
Abstract
This study presents a case of a 72-year-old man diagnosed with non-small cell lung cancer (cT4N0M0) referred to our hospital for possible surgical treatment of a solitary nodule detected in the mesorectum. The patient had received combined chemoradiotherapy and achieved a complete response 13 months before the presentation. On examination, the mesorectal nodule was incidentally detected during surveillance computed tomography, and the maximum standardized uptake value of the nodule was 10.3. Because of the potential malignancy and need for en-bloc resection of the nodule, we performed laparoscopically assisted high anterior resection of the rectum. The postoperative course was uneventful. Notably, while pathological examination revealed that the mesorectal nodule comprised an intravenous organized thromboembolism, malignancy was not observed. These findings suggest that although positron emission tomography/computed tomography with 18F-fluorodeoxyglucose is useful for the diagnosis of malignant diseases, surgical resection might be the most reliable option for complex cases such as ours.
Introduction
Positron emission tomography (PET)/computed tomography (CT) using 18F-fluorodeoxyglucose (FDG) is extensively utilized for diagnosing and staging malignant diseases. However, since FDG uptake is indicative of glucose metabolism activity, it is not exclusively specific to malignant diseases and can frequently be observed in benign pathological conditions such as infections or inflammatory lesions [1, 2]. Herein, we present a rare case of a patient with non-small cell lung cancer (NSCLC) who underwent surgery for a solitary thromboembolism in the mesorectum, which showed a high maximum standardized uptake value (SUVmax) on FDG PET/CT scan.
Case report
A 72-year-old man diagnosed with NSCLC was referred for possible surgical treatment of a solitary nodule detected in the mesorectum. His medical history included hypertension and endoscopic resection of colonic polyps, with no prior thromboembolism. He had received combined medical treatment for NSCLC (cT4N0M0, stage IIIA) (Fig. 1), including four courses of cisplatin and docetaxel chemotherapy with a 60 Gy/30 fractions radiation dose, followed by 1 year of consolidation therapy with durvalumab (640 mg/body). The patient tolerated the treatment well, achieving a complete response 13 months before presentation (Fig. 2). However, the mesorectal nodule was incidentally discovered during surveillance CT after NSCLC treatment completion. It was round, 15 mm in diameter, showed contrast enhancement, and was near the mesorectal vessels (Fig. 3a and b). An FDG PET/CT scan revealed a solitary mesorectal nodule with an SUVmax of 10.3 (Fig. 4). Consequently, the radiologist suggested differential diagnoses of malignant lymphoma and metastatic lymph nodes from the urinary or lower gastrointestinal tract, as NSCLC typically does not metastasize to mesorectal lymph nodes. No other abnormal FDG uptake was observed. Laboratory tests showed normal levels for tumor markers, including carcinoembryonic antigen, sialyl Lewis X (SLX), squamous cell carcinoma antigen, neuron-specific enolase, cytokeratin fragment (CYFRA), progastrin-releasing peptide, and blood coagulability was within the normal range. Total colonoscopy revealed no neoplastic lesions, and urinary cytology showed nonmalignant urothelial cells. Noninvasive diagnostic approaches, including endoscopic or CT-guided biopsy, were extensively discussed but deemed difficult because of anatomical restrictions, risk of dissemination, and procedure-related complications (such as bleeding or perforation). Because of the potential malignancy and need for en-bloc resection, we opted for surgical resection using a standardized laparoscopically assisted mesorectal excision technique. During rectal dissection, the nodule was not visible through the posterior and lateral sides because it was completely embedded in the mesorectum. Therefore, a Pfannenstiel incision was made in the lower abdomen to exteriorize the rectum after the division of the proximal colon. The nodule was confirmed by direct palpation, marked with a stitch, and subsequently removed after intracorporeal transection of the distal rectum. We inspected the resected specimen and confirmed that the nodule was incorporated (Fig. 5a and b). A colorectal anastomosis was then performed using a double-stapling technique with a circular stapler. The postoperative course was uneventful, except for a slight elevation of the d-dimer level (up to 3.26 μg/ml) on postoperative Day 7, which normalized spontaneously without intensive anticoagulation therapy. The patient was discharged on postoperative Day 10. Pathological examination revealed that the 7-mm white nodule was an intravenous organized thrombus in the mesorectum surrounded by granulation tissue, with no malignancy observed (Fig. 6a and b). Postoperatively, the patient did not require additional chemotherapy or anticoagulation therapy. During the 16-month follow-up, no radiological evidence of NSCLC recurrence and thromboembolism was detected (Fig. 7).
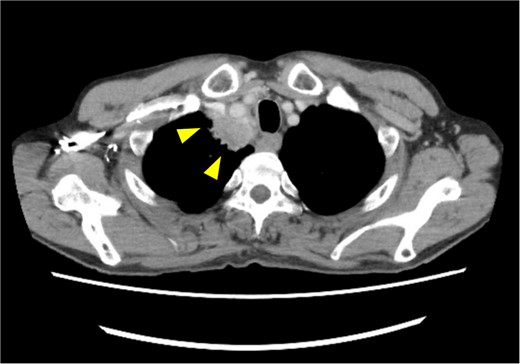
Pretreatment chest computed tomography (CT) image. The arrowheads highlight locally advanced lung cancer invading the chest wall and brachiocephalic trunk (cT4N0M0).
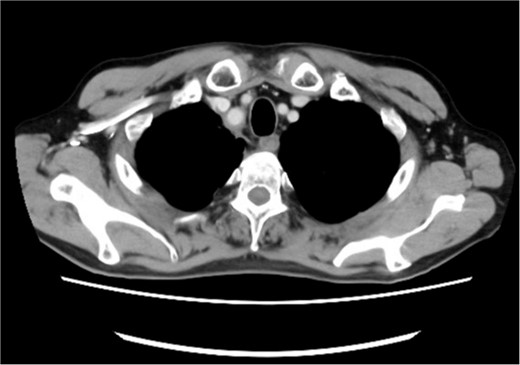
Posttreatment computed tomography (CT) image. No apparent tumorous lesions were identified.
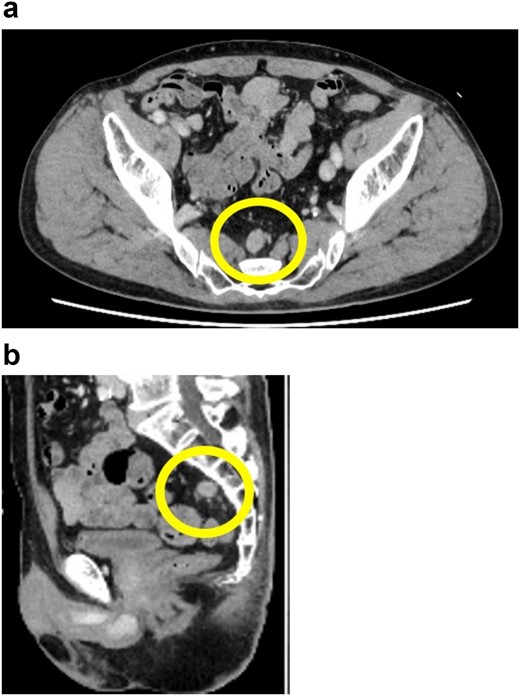
Preoperative pelvic computed tomography (CT) image. The circle indicates the nodule located in the mesorectum. (a) Horizontal view. (b) Sagittal view.
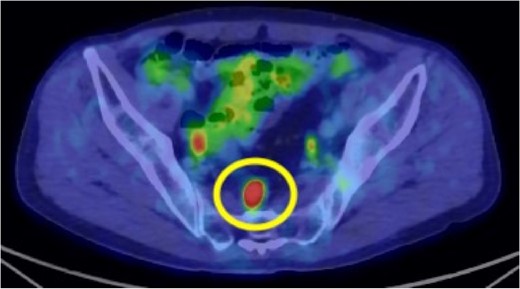
Preoperative 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) image. The circle highlights the nodule located in the mesorectum with a maximum standardized uptake value (SUVmax) of 10.3.
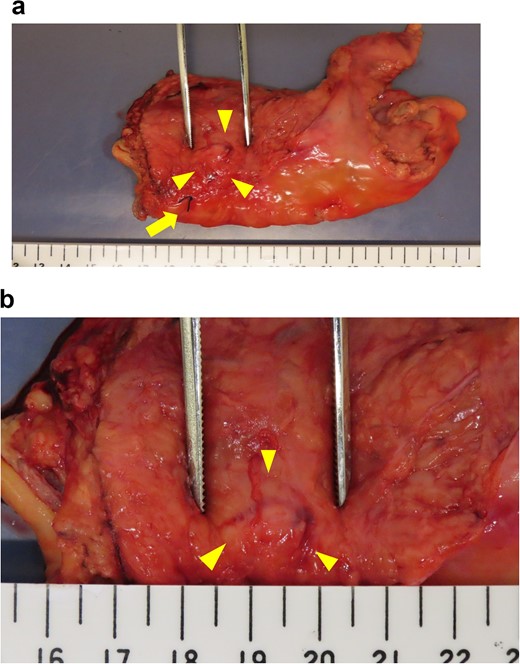
Intraoperative evaluation of the resected specimen. The specimen was removed by tumor-specific mesorectal excision. The arrowheads indicate the area containing the nodule. The arrow indicates the marking stitch placed at the distal edge of the nodule. (a) View of the specimen. (b) Enlarged view of the nodule.
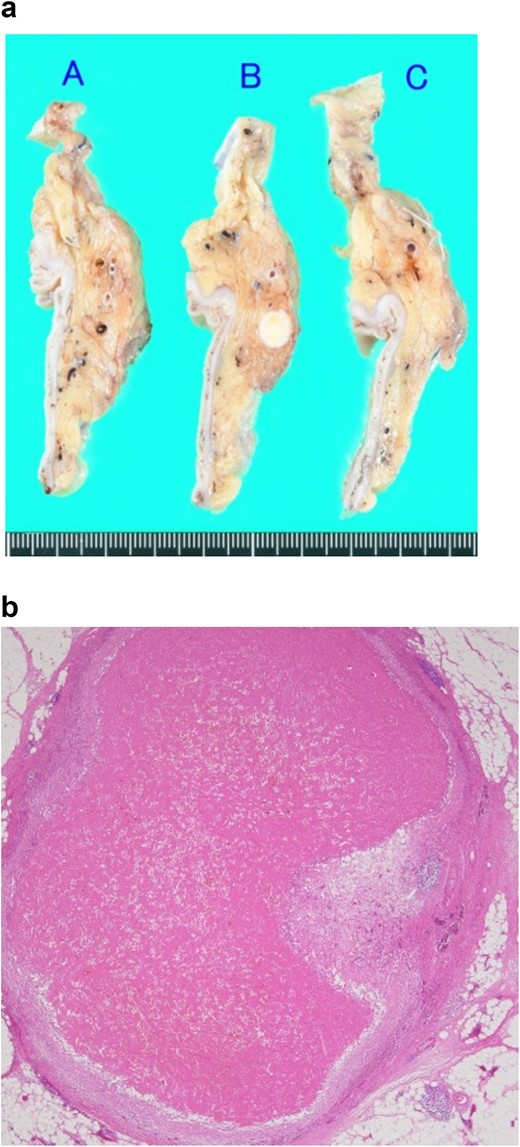
Pathological findings of the resected specimen. (a) Macroscopic finding. A solid white nodule with a diameter of 7 mm is embedded in the mesorectum. (b) Microscopic findings observed with hematoxylin–eosin staining. The nodule comprises an intravenous organized thrombus with surrounding granulation tissue. No malignancy is observed.
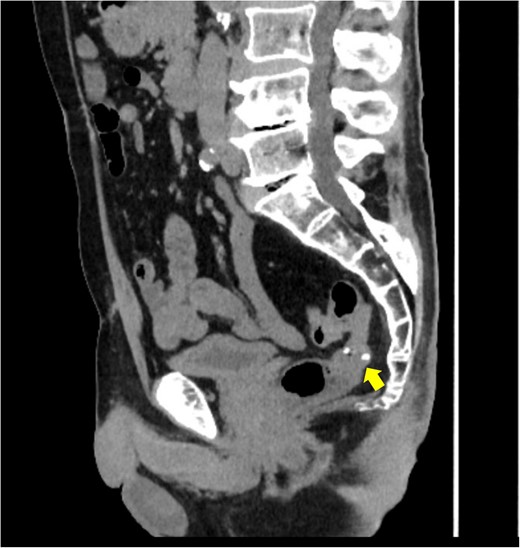
Surveillance computed tomography (CT) image of the pelvis at 16 months postoperatively. No lesions were observed in the mesorectum. The arrow indicates the staples used at the colorectal anastomosis.
Discussion
Distant metastasis of NSCLC to the abdominal lymph nodes is rare [3, 4], with an incidence rate of 6% (74 of 1192 cases), and only 10 reported cases have been upgraded to stage IV following the identification of abdominal lymph node metastasis [5]. A retrospective study of distant metastasis due to NSCLC published in 2021 found that 68 of 2827 patients who underwent surgical resection of stage I NSCLC developed recurrence within 1 year of surgery. The recurrence sites were the bone (30%), liver (30%), brain (28%), and adrenal gland (12.5%) [6]. However, due to the rarity of NSCLC metastasis to the abdominal lymph node, the detected nodule in our case could not be confidently considered lymph node metastasis of NSCLC.
FDG PET/CT is widely used for clinical staging of malignancy and detecting postoperative recurrence due to its ability to provide relevant metabolic information. It is well-known that SUVmax is higher for malignant lesions compared to benign ones, reflecting their active glucose metabolism. A previous study showed that FDG PET/CT has good diagnostic performance for staging distant metastasis in patients with NSCLC [7]. Additionally, SUVmax is useful for diagnosing venous thromboembolism (VTE) [8]. Several studies have demonstrated the efficacy of FDG PET/CT in distinguishing venous tumor thrombus from venous bland thrombus. Hu et al. [9] reported that tumor embolisms associated with portal vein thrombosis had a significantly higher SUVmax than simple thrombi, with tumor embolisms showing an SUVmax of 6.37 ± 2.67 and simple thrombi showing an SUVmax of 2.87 ± 1.47. Similar results were found in studies on renal cell carcinoma and hepatocellular carcinoma [10–12]. In our present case, the SUVmax of the mesorectal nodule was 10.3, which reasonably led to suspicion of malignancy.
The underlying mechanism of the solitary mesorectal thromboembolism in this case remains unclear. Patients with malignant diseases are at high risk for VTE, and cancer-associated thrombosis is a major complication among patients with lung cancer [13]. The risk of cancer-associated thrombosis is influenced by patient and cancer characteristics. For instance, conditions such as anemia, chronic obstructive pulmonary disease, and obesity can increase the risk of VTE [14]. Additionally, chemotherapy is an independent risk factor for VTE in patients with lung cancer [13]. A large retrospective study published in 2011 reported that 18.1% of patients treated with cisplatin-based chemotherapy experienced thromboembolic events during treatment or within 4 weeks of the last treatment dose [15]. While our patient did not have comorbidities associated with risk factors for VTE, we posit that cisplatin-based chemotherapy might have contributed to the development of thromboembolism. To the best of our knowledge, no previous studies have reported solitary thromboembolism in the mesorectum. Notably, given the high SUVmax of the nodule during FDG PET/CT, it was challenging to rule out malignancy in our case.
In conclusion, although FDG PET/CT is considered highly useful for diagnosing malignant lesions, surgical resection may be the most reliable option for both the diagnosis and treatment of complex cases like ours. Moreover, it is crucial to employ a safe and minimally invasive approach.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Yoshihiro Sato and Shingo Tsujinaka. The first draft of the manuscript was written by Yoshihiro Sato, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Human rights
The authors declare that this study conformed to the Declaration of Helsinki.
Informed consent
Informed consent was obtained from the patient for publication of this case report and any accompanying images.



