-
PDF
- Split View
-
Views
-
Cite
Cite
Brady J Anderson, Zachary G Tanenbaum, Alexander Michael, Scott R Owen, Cutaneous hypersensitivity to chlorhexidine following facial fat grafting: a delayed intradermal response, Journal of Surgical Case Reports, Volume 2024, Issue 7, July 2024, rjae103, https://doi.org/10.1093/jscr/rjae103
Close - Share Icon Share
Abstract
Chlorhexidine (CHD) is commonly included in surgical antiseptics and can be associated with adverse reactions ranging from contact dermatitis to anaphylaxis. A 32-year-old female presented to the OR for facial fat grafting. Surgical sites were prepped with CHD gluconate or topical iodine. Donor and recipient sites were infiltrated with local anesthetic injection prior to fat harvest and facial injection. Eleven days later, she presented with new painful, pruritic rash over donor sites where CHD had been applied prior to local anesthetic infiltration. Treatment with topical clobetasol and prednisone taper resulted in complete symptom resolution. This patient’s response most likely represented a delayed type IV, T-cell mediated hypersensitivity. CHD is a known trigger of allergic reactions. Infiltration of local anesthetic may introduce skin prep into the subcutaneous tissue akin to intradermal testing. For those with delayed cutaneous reactions, steroids may provide symptomatic relief.
Introduction
Facial fat grafting (FFG) involves autologous fat harvest followed by injection into pre-determined sites for augmentation or contouring and is performed for both cosmetic and reconstructive purposes [1]. Before beginning the fat harvest, donor and recipient areas are cleansed with surgical antiseptic solution and infiltrated with tumescent solution containing local anesthetic and hemostatic agents.
Chlorhexidine (CHD) is a common antiseptic found in surgical prep solutions and over-the-counter cosmetic products. Allergy rates are estimated at ~1% with reactions ranging from minor dermatitis to anaphylaxis [2–5]. Recently, increasing attention has been directed toward highlighting the allergenic potential of CHD and reducing patient exposure [5]. This case report demonstrates an example of moderately severe, delayed CHD allergy following planned FFG.
Case report
A 32-year-old woman allergic to penicillins and benzocaine-menthol presented for planned FFG. The abdomen, flank, and thighs were prepped with Chloraprep (CHD gluconate 2% w/v and isopropyl ethanol) while the face was prepped with a topical iodine solution. Donor and recipient sites were subsequently infiltrated with a mixture of tranexamic acid, lidocaine, bupivicaine, and epinephrine. Fat was extracted from the umbilicus, lower abdomen, and lateral thighs using a Coleman Microfat Transfer Cannula, then prepared and injected into the face uneventfully. She received one dose of intra-operative dexamethasone, and was discharged from recovery with Cephalexin, a 6-day methylprednisolone taper, and topical Bacitracin.
Eleven days later, the patient reported an eruption of new skin lesions over the lower abdomen and thighs (Fig. 1). After close examination, lower-abdominal lesions were correlated with sites of needle injection of tumescent solution inserted through skin coated with CHD. She denied any recent exposure and was no longer using bacitracin. She had no facial lesions, where iodine and the same anesthetic mixture were used. Despite administration of diphenhydramine, her pruritus continued, followed by an eruption of painful and pruritic papules along her upper abdomen a few days later (Fig. 2). At that time, she was prescribed topical Clobetasol, a prednisone taper, and a 14-day course of valacyclovir with good resolution of her symptoms.
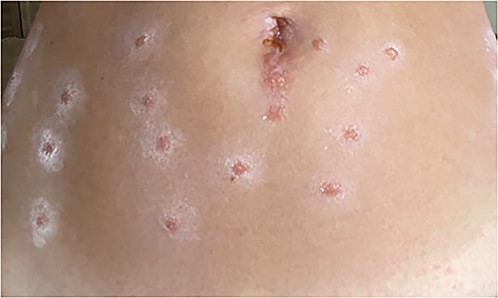
Lower abdomen with multiple scattered, roughly 1 cm, discreet well-circumscribed shiny papules with border of ill-defined ashy scale arranged in parallel vertical lines in locations of needle injection of tumescent solution inserted through skin coated with CHD.
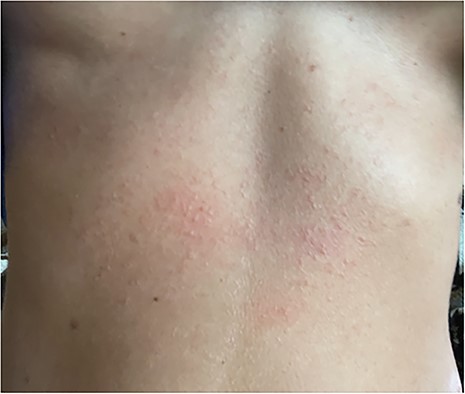
Upper abdomen with Ill-defined and erythematous eczematous papules coalescing into a poorly marginated thin plaque with mild background erythema.
Discussion
CHD allergy may be more common than previously believed, with one study demonstrating positive patch test to CHD in up to 1% of patients [2]. Adverse reactions to CHD range from cell-mediated type IV hypersensitivity dermatitis to immediate type I hypersensitivity manifesting as anaphylaxis or cardiac arrest [4–7]. CHD is included alongside latex, antibiotics, and certain paralytics as a common cause of perioperative anaphylaxis [7]. Even without subcutaneous infiltration, exposure to CHD in allergic individuals can be dangerous; in one report of perioperative CHD allergy, 26/53 (49%) patients with recorded exposure route were exposed by skin preparation alone [8].
In those with presumed CHD allergy, allergic testing may confirm the diagnosis [2, 8]. Skin patch testing, skin prick testing, intradermal testing (IDT), specific IgE testing, and basophil activation tests have all been reported for this purpose, although standardized protocols or gold standard provocation tests do not exist [9]. Reported quantities of CHD used for IDT include 20 μl of CHD, 5 mg/ml in 1:1000 with saline (0.005 mg/ml) and 20 μl of CHD, 0.002 mg/ml [8, 9]. However, these reports have focused on immediate reading of wheal following IDT (type I hypersensitivity). Delayed readings following IDT may be required to detect cell-mediated reactions [10].
At 11 days post-exposure, our patient’s response most likely represented a delayed type IV, T-cell mediated hypersensitivity. The CHD painted onto her skin was infiltrated into the subcutaneous tissue during injection of local anesthesia, effectively performing an IDT of the CHD, with the iodine prep used on the face as a negative control. It is possible that the intra-operative steroid dose and postoperative steroid taper delayed the presentation of her symptoms, as steroids blunt the T-cell mediated immune response and can interfere with results of testing for delayed hypersensitivity [11]. Her symptoms responded to management with oral and topical steroids, the typical treatments for contact dermatitis [6]. However, had she been sensitized with significant quantities of IgE, intravascular infiltration could have precipitated an immediate type I hypersensitivity reaction with potentially devastating results [3, 4].
Outside the hospital, CHD is found in various over-the-counter creams and products, of which facial plastic surgery patients may be long-time users [5]. Whether use of these products may sensitize individuals to CHD allergy has not been studied extensively, but they could theoretically constitute a source for re-exposure in affected individuals. At the systemic level, improved labeling of CHD content (akin to latex) has been suggested to decrease accidental exposure or re-exposure to CHD by patients or providers [5]. In addition, a diligent allergy history, including adverse responses to surgical materials, should be seen not as a checklist item, but a chance to screen patients for complications to otherwise low-risk procedures.
Conclusion
CHD is a known trigger of allergic reactions in sensitive individuals, ranging from delayed skin lesions to immediate anaphylaxis. Infiltration of local anesthetic may introduce skin prep into the subcutaneous tissue akin to IDT. For those with delayed cutaneous reactions, steroids may provide symptomatic relief.
Acknowledgements
We appreciate the support from the University of Iowa Hospitals and Clinics and the other hospital staff who took part in this patient’s care as well as our research initiatives.
Author contributions
Brady J. Anderson: manuscript drafting; Zachary Tanenbaum: manuscript revision, direct patient care; Alexander Michael: direct patient care; Scott R. Owen: project conception, data oversight, manuscript revision, direct patient care.
Conflict of interest statement
The authors declare they have no conflict of interest.
Funding
Publication fee funded by the Department of Otolaryngology, University of Iowa Hospitals and Clinics.
References
Author notes
This article was presented as a poster at COSM AAFPRS 2024.



