-
PDF
- Split View
-
Views
-
Cite
Cite
Masoud Akbari, Avram Alter, Keith A Kuenzler, Spindle cell sarcoma of the chest wall: a pediatric case report, Journal of Surgical Case Reports, Volume 2024, Issue 6, June 2024, rjae431, https://doi.org/10.1093/jscr/rjae431
Close - Share Icon Share
Abstract
Chest wall sarcomas are reported to be infrequent among thoracic tumors. The spindle cell subtype makes up a small percentage of this group. These tumors can be asymptomatic or cause symptoms of chest pain and shortness of breath due to the mass effect, which can lead to a delay in diagnosis. A 10-year-old female with a persistent cough, shortness of breath on exertion, and left-sided chest pain presented to the ED. Imaging indicated a chest wall mass filling the left hemithorax with a rightward mediastinal shift. During surgical resection, two tumors were removed, with resection of parts of the latissimus dorsi and serratus anterior. A diagnosis of MGA:NUTM1 spindle cell sarcoma was made pathologically. The patient was successfully treated with surgery and adjuvant chemoradiotherapy. We hope to add to our academic knowledge by presenting the presentation and treatment of SCS in a pediatric patient.
Introduction
Soft tissue sarcoma (STS) is a rare tumor, representing less than 1% of all reported tumors [1]. STSs make up 10%–15% of primary chest wall tumors, excluding primary breast malignancies. Spindle cell sarcoma (SCS) is an STS subtype, so rare that there are no reported statistics [1–4]. SCS presents differently in various organs and shows different histopathological features. The median age of diagnosis is 61, with 80.9% of cases occurring in Caucasians [5]. This paper discusses the presentation and management of SCS in a pediatric patient.
Case report
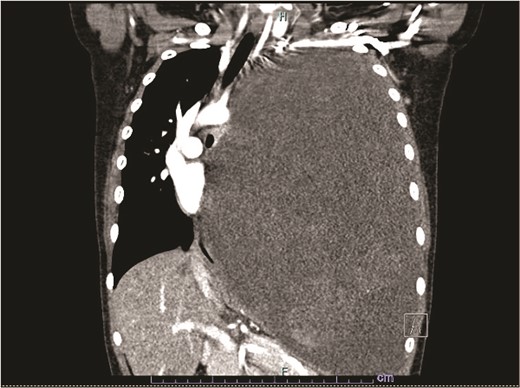
Our patient is a 10-year-old Caucasian female with a history of early adrenarche, ADHD, and COVID-19 and no past surgical history. She presented to the ED with a month-long history of persistent coughing, shortness of breath on exertion and speaking, and left-sided chest pain that began after a diagnosis of mildly symptomatic COVID-19. A physical exam revealed decreased breath sounds on the left, with a left-sided chest protrusion. A plain film and CT demonstrated a normal right lung, with a significant mediastinal shift toward the right, displacement of the heart into the right hemithorax, with complete whiteout of the left hemithorax, and demonstrating a mix of solid and cystic foci, with a round opacification at the left lung base (Figs 1 and 2). The patient underwent an uncomplicated left thoracotomy with excision of two masses, one being 22.5 × 21.0 × 10.5 cm and the other being 13.5 × 11.5 × 6.2 cm. These masses weighed 2394 g in aggregate. The specimens consisted of well-encapsulated tumors attached to the posterior aspect of the chest wall. A regional, positive lymph node measuring 3.0 × 2.2 × 1.0 cm was also resected. Due to the large size of the tumor and the requirement for clean margins, parts of the latissimus dorsi and serratus anterior muscles were also resected. By postoperative day (POD) 0, the left lung had already filled the left hemithorax, and the mediastinal shift had corrected (Fig. 3). The chest tube was removed on POD 5, and a plain film showed continued expansion of the lung to full size and resolving pulmonary edema and atelectasis (Fig. 4). The patient was discharged home on POD 5. The tumor staging was T4N1M0. The tumor was positive for vimentin, CD56, synaptophysin, chromogranin, and NSE. The tumor was negative for pancytokeratin, Cam5.2, EMA, S100, desmin, SMA, SOX10, HMB45, CD117, DOG1, CD99, PR, CD68, WT1, CD30, p53, Alk1, GFAP, and nuclear β-catenin. Next-generation sequencing showed an MGA:NUTM1 fusion, supporting a final diagnosis of high-grade MGA:NUTM1 fusion SCS. Given the size and grading of the tumor, the patient underwent adjuvant CT-RT with ifosfamide, doxorubicin, and proton beam radiotherapy, with supportive doses of MESNA and dexrazoxane for 4.5 months post-operatively. The basis of this treatment is ARST0332 Arm C [6]. Before treatment initiation, the patient underwent an uncomplicated left oophorectomy for reproductive cryopreservation. A PET/CT prior to CT-RT showed hypermetabolic activity in several areas. These included the pleural resection margin, a mediastinal lymph node concerning for metastasis versus postsurgical change, and a left axillary lymph node concerning for metastatic disease. During treatment, the patient contracted human metapneumovirus and rhino/enterovirus, resulting in febrile neutropenia. The patient was successfully treated with blood and platelet transfusions and pegfilgrastim. Upon completion of CT-RT, PET/CT imaging showed no evidence of residual disease, with minimal focal hypermetabolic activity as compared to the previous imaging (Fig. 5).
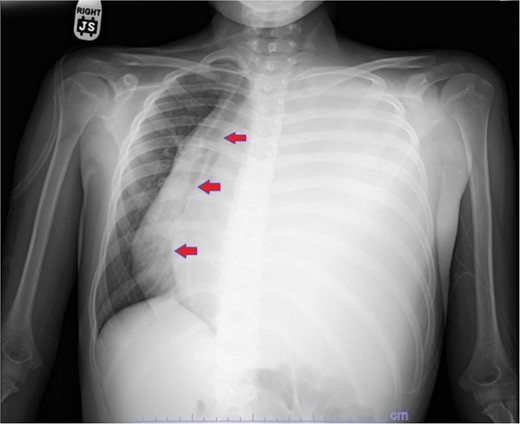
Plain film showing complete left hemithorax opacification and mediastinal shift (arrows) to right.
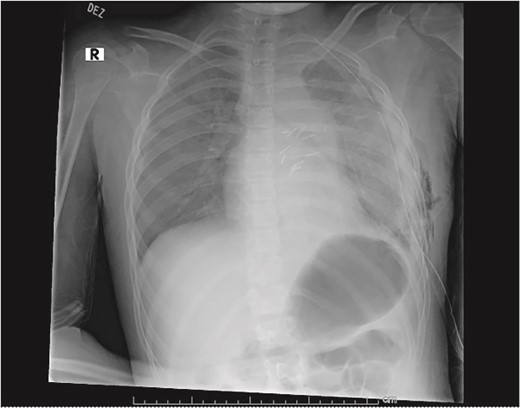
Plain film on postoperative day 0 depicting the recovery of the left lung post-surgical resection.
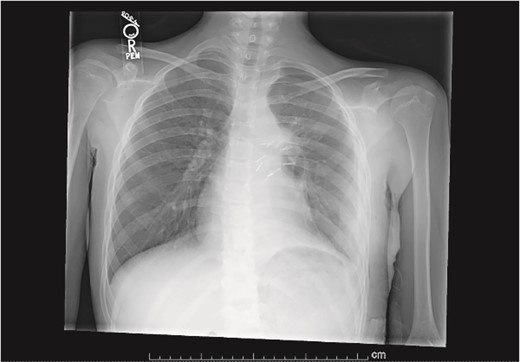
Plain film postoperative day 5, after removal of chest tube, showing improving pulmonary status in the left hemithorax.
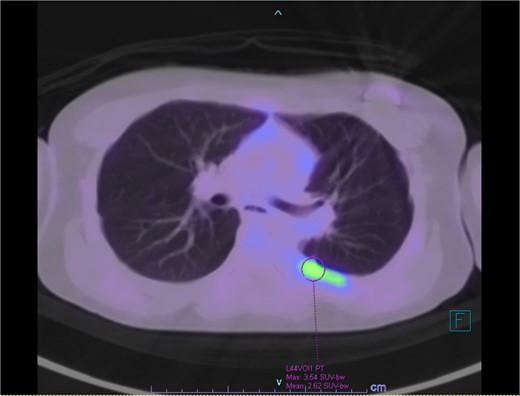
PET/CT showing residual hypermetabolic focus at plural resection margin, concerning for residual disease.
Discussion
Chest wall sarcomas are slow-growing, indolent tumors that are challenging to diagnose due to highly variable findings. Of the thoracic malignancies, chest wall masses comprise less than 5%, with a malignancy rate of 50%, and can be primary or metastatic. More than 20% of chest wall tumors are found incidentally on imaging, and if symptomatic, pain is the most common presentation in both benign and malignant cases [7]. At the time of diagnosis, most tumors have grown quite large, greater than 15 cm in diameter [1]. The mainstay of treatment is surgical resection, with adjuvant chemotherapy and radiation used in specific scenarios [8]. In a single-institution retrospective study, Burt et al. showed that there was a significant improvement in 5- and 10-year disease-free survival between those who received chemotherapy (improvement of 82%) or radiotherapy (improvement of 92%), and those who received both (89% at 5-year and 90% at 10-year), than in those treated with surgical resection alone. They also noted a significant mortality benefit (reduction to 49% 5-year and 45% 10-year) compared to those treated only with surgery [9]. Depending on tumor characteristics like histology and grade, surgical resection of margins, and patient sex, chest wall sarcomas have a 5-year survival rate of 59%–68%, with surgical resection the mainstay of treatment [10].
There are over 100 different types of sarcomas with different biomarkers [11]. A correct diagnosis is imperative, as the tumor subtype guides patient management, treatment, and prognosis [11, 12]. Biomolecular markers have been increasingly utilized to form diagnoses, as specific markers have been found consistently in subtypes of sarcoma. These tumor markers show a wide range of derangements, including overexpression, epigenetic regulation, inactivation, amplification, or gene fusion. MGA:NUTM1 gene fusion has variable expression of several markers, like CD99, CD34, and BCL2, and is negative for cytokeratins, p63/p40, EMA, S100, or GFAP [13]. NUTM1 has been linked to poorly differentiated tumors called NUT-associated carcinomas [13, 14]. It is thought that the transcription of the MGA:NUTM1 fusion gene is driven by the MGA gene promoter, and the NUTM1-rearranged protein drives the malignant transformation of mesenchymal tissue into high-grade sarcoma [15].
Conclusion
Chest wall SCSs are rare tumors that require a multidisciplinary approach to treatment. Surgical resection is the mainstay of treatment. Adjuvant radiation and chemotherapy also play important roles. Adjuvant therapy depends on tumor subtype and markers, and the extent of the disease. Additionally, recognizing the potential occurrence of this carcinoma subtype outside of the anticipated demographics or age range can help expedite the correct diagnosis and treatment with favorable outcomes.
Funding
None declared.
References
- chest pain
- immunologic adjuvants
- pharmaceutical adjuvants
- pediatrics
- sarcoma
- surgical procedures, operative
- thoracotomy
- diagnosis
- diagnostic imaging
- neoplasms
- surgery specialty
- soft tissue sarcomas
- chest wall
- mediastinal shift
- exertional dyspnea
- chest wall mass
- serratus anterior muscle
- latissimus dorsi muscle
- excision
- spindle-cell sarcoma



