-
PDF
- Split View
-
Views
-
Cite
Cite
Richi Nakatake, Hiroaki Kitade, Morihiko Ishizaki, Hidesuke Yanagida, Tetsuya Okuyama, Yoshiko Uemura, Mitsugu Sekimoto, Giant hemorrhagic pancreatic pseudocyst with suspected cystic pancreatic tumor: a case report, Journal of Surgical Case Reports, Volume 2024, Issue 6, June 2024, rjae393, https://doi.org/10.1093/jscr/rjae393
Close - Share Icon Share
Abstract
Pancreatic pseudocysts are surrounded by a non-epithelialized wall confined to the pancreas and localized to the pancreatic tissue or adjacent pancreatic cavity. In contrast, pancreatic cystic tumors occur less frequently than solid lesions and are often detected incidentally on imaging. Regarding the qualitative diagnosis of pancreatic pseudocysts, it is important to differentiate them from neoplastic cysts. We report the case of a 74-year-old woman with a giant hemorrhagic pancreatic pseudocyst and a suspected cystic pancreatic tumor, wherein distal pancreatectomy and splenectomy with lymph node dissection were performed. The patient was discharged 11 days postsurgery, with a good postoperative course. There are no reports of giant pancreatic pseudocysts larger than 10 cm with hematoma contents. The presumptive diagnosis of pseudocysts based on imaging alone may be difficult. Surgical resection is considered when it is difficult to distinguish a giant pancreatic pseudocyst from a cystic neoplasm.
Introduction
Pancreatic pseudocysts are surrounded by a non-epithelialized wall confined to the pancreas and localized in the pancreatic tissue or adjacent pancreatic cavity. Pancreatic pseudocysts are primarily a complication of pancreatitis or pancreatic trauma [1]. Pancreatic pseudocysts are often asymptomatic but rarely associated with abdominal pain or other symptoms.
In contrast, pancreatic cystic tumors occur less frequently than solid lesions and are often detected incidentally with imaging. In the qualitative diagnosis of pancreatic pseudocysts, differentiation from neoplastic cysts is important [2]. However, it is very difficult to differentiate non-neoplastic cystic lesions from neoplastic lesions based on imaging criteria alone [3]. Herein, we report a case of a giant hemorrhagic pancreatic pseudocyst resected with a suspected pancreatic cystic tumor.
Case presentation
A 74-year-old Asian woman had been treated for diabetes mellitus but had stopped treatment on her own. The HbA1c level increased sharply to 16%. Blood samples showed no abnormal findings such as elevated levels of tumor markers (Table 1). There was no history of abdominal imaging, sudden abdominal pain, abdominal trauma, or abdominal surgery. Contrast-enhanced abdominal computed tomography (CT) revealed a large hematoma-like cystic lesion in the pancreatic tail (Fig. 1a–c). Calcification of the wall and internal septum was prominent. Magnetic resonance imaging (MRI) demonstrated a 156 × 112 mm marginal T1W high signal (Fig. 2a) and an internal T2W uneven low signal (Fig. 2b) on the dorsal side of the stomach, suggesting a hematoma-like cystic lesion. Diffusion showed a high signal intensity, but abnormal enhancement was not observed (Fig. 2c). Considering the possibility of intra-abdominal dissemination of the cystic tumor via a puncture, the patient underwent distal pancreatectomy and splenectomy with lymph node dissection for diagnostic and therapeutic purposes. The cyst wall adhered strongly to the surrounding tissue (Fig. 3); however, no adverse events were noted during the operation. The operating time was 7 h 19 min, and blood loss was 809 ml. The patient was discharged 11 days postoperatively, with a good postoperative course.
| WBC | 6500 | /μl |
| RBC | 570 | /μl |
| Hb | 14.9 | g/dl |
| Plt | 13.8 | μl |
| PT | 108 | % |
| TP | 8.2 | g/dl |
| Alb | 4.1 | g/dl |
| BUN | 150 | smg/dl |
| Cre | 0.84 | mg/dl |
| Na | 135 | mmol/l |
| K | 4.5 | mmol/l |
| Cl | 99 | mmol/l |
| AST | 18 | U/l |
| ALT | 16 | U/l |
| ALP | 92 | U/l |
| LDH | 141 | U/l |
| T-Bil | 0.7 | mg/dl |
| D-Bil | 0.2 | mg/dl |
| AMY | 63 | U/l |
| γ-GTP | 14 | U/l |
| ChE | 313 | U/l |
| CRP | 0.05 | mg/dl |
| HbA1c | 16.4 | % |
| CA19-9 | 26.8 | U/ml |
| CEA | 4 | ng/ml |
| WBC | 6500 | /μl |
| RBC | 570 | /μl |
| Hb | 14.9 | g/dl |
| Plt | 13.8 | μl |
| PT | 108 | % |
| TP | 8.2 | g/dl |
| Alb | 4.1 | g/dl |
| BUN | 150 | smg/dl |
| Cre | 0.84 | mg/dl |
| Na | 135 | mmol/l |
| K | 4.5 | mmol/l |
| Cl | 99 | mmol/l |
| AST | 18 | U/l |
| ALT | 16 | U/l |
| ALP | 92 | U/l |
| LDH | 141 | U/l |
| T-Bil | 0.7 | mg/dl |
| D-Bil | 0.2 | mg/dl |
| AMY | 63 | U/l |
| γ-GTP | 14 | U/l |
| ChE | 313 | U/l |
| CRP | 0.05 | mg/dl |
| HbA1c | 16.4 | % |
| CA19-9 | 26.8 | U/ml |
| CEA | 4 | ng/ml |
| WBC | 6500 | /μl |
| RBC | 570 | /μl |
| Hb | 14.9 | g/dl |
| Plt | 13.8 | μl |
| PT | 108 | % |
| TP | 8.2 | g/dl |
| Alb | 4.1 | g/dl |
| BUN | 150 | smg/dl |
| Cre | 0.84 | mg/dl |
| Na | 135 | mmol/l |
| K | 4.5 | mmol/l |
| Cl | 99 | mmol/l |
| AST | 18 | U/l |
| ALT | 16 | U/l |
| ALP | 92 | U/l |
| LDH | 141 | U/l |
| T-Bil | 0.7 | mg/dl |
| D-Bil | 0.2 | mg/dl |
| AMY | 63 | U/l |
| γ-GTP | 14 | U/l |
| ChE | 313 | U/l |
| CRP | 0.05 | mg/dl |
| HbA1c | 16.4 | % |
| CA19-9 | 26.8 | U/ml |
| CEA | 4 | ng/ml |
| WBC | 6500 | /μl |
| RBC | 570 | /μl |
| Hb | 14.9 | g/dl |
| Plt | 13.8 | μl |
| PT | 108 | % |
| TP | 8.2 | g/dl |
| Alb | 4.1 | g/dl |
| BUN | 150 | smg/dl |
| Cre | 0.84 | mg/dl |
| Na | 135 | mmol/l |
| K | 4.5 | mmol/l |
| Cl | 99 | mmol/l |
| AST | 18 | U/l |
| ALT | 16 | U/l |
| ALP | 92 | U/l |
| LDH | 141 | U/l |
| T-Bil | 0.7 | mg/dl |
| D-Bil | 0.2 | mg/dl |
| AMY | 63 | U/l |
| γ-GTP | 14 | U/l |
| ChE | 313 | U/l |
| CRP | 0.05 | mg/dl |
| HbA1c | 16.4 | % |
| CA19-9 | 26.8 | U/ml |
| CEA | 4 | ng/ml |
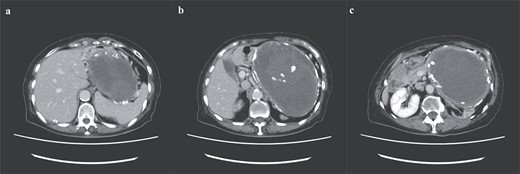
Preoperative abdominal enhanced CT showing a large hematoma-like cystic lesion in the pancreatic tail.
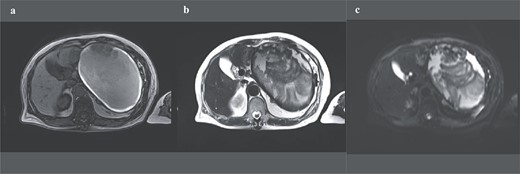
Preoperative abdominal enhanced MRI showing a 156 × 112 mm marginal T1W high (a) and an internal T2W uneven low signal (b); diffusion shows some high signal, but abnormal enhancement cannot be noted (c).
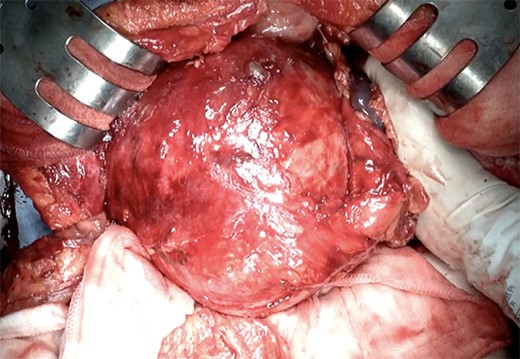
The intraoperative photograph of a giant pancreatic cystic lesion.
Grossly, the excised specimen consisted of the pancreatic parenchyma and a large cystic mass measuring 16 × 17 × 13 cm (Fig. 4a), which was a unilocular cyst without intraluminal protrusions and filled with hemorrhagic and thrombus-like contents (Fig. 4b). Microscopically, a duct with intraductal epithelial hyperplasia similar to low-grade pancreatic intraepithelial neoplasia (PanIN) was observed in the pancreatic parenchyma; however, obvious neoplastic lesions were not detected. The cyst wall was composed of hyalinized fibrous tissue with prominent calcification. However, no intraluminal epithelial lining or pancreatic parenchyma was observed. The continuity of the cystic lumen and pancreatic parenchyma was not detected; therefore, this long-standing hematoma-like lesion was considered a pancreatic pseudocyst (Fig. 4c).

Macroscopic (a, b) and histopathological findings (c); (a) the excised specimen consisted of pancreatic parenchyma and a huge cystic mass measuring 16 × 17 × 13 cm; (b) the huge cystic mass was unilocular cyst without intraluminal protrusions and filled with hemorrhagic and thrombus-like contents; (c) hematoxylin–eosin staining, from left to right: pancreatic tissue, cyst wall (eosinophilic zone), cyst lumen; no obvious neoplastic lesions were detected within the pancreatic parenchyma; the cyst wall was composed of hyalinized fibrous tissue with marked calcification, but no intraductal epithelial lining or pancreatic parenchyma was observed; the continuity of cystic lumen and pancreatic parenchyma was not detected, therefore this long-standing hematoma like lesion was considered pancreatic pseudocyst.
Discussion
The American Gastroenterological Association published the guidelines for the diagnosis and management of pancreatic cysts in 2018. MRI or MR cholangiopancreatography is the imaging modality of choice for confirming the diagnosis of a pancreatic pseudocyst, which is usually followed by ultrasound (US) noting an encapsulated mass; for patients who cannot undergo MRI, CT, or US are options. Finally, undetermined cysts may undergo endoscopic US with fine needle aspiration for analysis of the cystic fluid [4]. Lack of specific clinical symptoms and laboratory findings, and overlap of imaging findings between non-neoplastic and neoplastic cystic lesions in pancreatic cystic tumors are often present [5]. Most pancreatic cysts are non-neoplastic, but ~10% are cystic tumors ranging from benign to highly malignant [6]. Most (up to 90%) clinically observed pancreatic cystic lesions are postinflammatory pseudocysts [7]. A presumptive diagnosis of pseudocysts based on imaging alone might be difficult in up to 50% of cases [8].
There are no reports in the MEDLINE database of large pancreatic pseudocysts with hematomas similar to this case. In pancreatitis and pancreatic trauma, intrapancreatic hemorrhage occurs due to the destruction of the peripancreatic vasculature by the release of pancreatic enzymes [9]. A history of cases suggestive of peripancreatic hemorrhagic disease usually assumes sudden abdominal pain, abdominal trauma, or a history of abdominal surgery that suggests or causes bleeding; however, this case is different. In this case, peripancreatic vascular and intrapancreatic hemorrhages, which developed either before or after pseudopancreatic cyst formation, were considered to represent the formation of a chronic dilated hematoma after spontaneous hemostasis.
Pancreatic pseudocysts vary in size from small (<2 cm) to medium (2–6 cm) and large. Several cases of giant pancreatic pseudocysts have already been reported in the literature; however, few cases of giant pancreatic pseudocysts with a maximum diameter of more than 10 cm have been reported in the literature, including details [1, 10, 11]. The three principal treatments used for pancreatic pseudocysts are pseudocyst drainage, vessel thromboembolization, and conservative management [12]. Surgical resection is indicated unless malignancy can be unequivocally excluded [13–15]. As in our case, there is a report of a patient who underwent pancreatectomy for a large pancreatic pseudocyst larger than 10 cm because of the difficulty in differentiating it from a pancreatic neoplasm [10].
Conclusion
Giant hemorrhagic pancreatic pseudocysts larger than 10 cm are rare. If a giant pancreatic pseudocyst cannot be differentiated from a cystic neoplasm, surgical resection is considered.
Author contributions
R.N. wrote the manuscript. R.N., M.I., and H.Y. were in charge of treatment of this case. H.K., T.O., Y.U., and M.S. reviewed the manuscript. All authors read and approved the final manuscript.
Conflict of interest statement
The authors have no conflict of interest to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors.
Ethical approval
Written informed consent for publication of their clinical details and clinical images was obtained from the patient.



