-
PDF
- Split View
-
Views
-
Cite
Cite
Mohammed Abdulmohsen AlSharit, Reem Mohammed Althwanay, Abdullah Saleh AlQattan, Abdulrahman Abdullah AlTamimi, Nabeel Mansi, Ectopic ampulla of vater in D4 with adenosquamous carcinoma: case report and literature review, Journal of Surgical Case Reports, Volume 2024, Issue 6, June 2024, rjae377, https://doi.org/10.1093/jscr/rjae377
Close - Share Icon Share
Abstract
Ampulla of Vater (AOV) is typically located in the second part of the duodenum. There are few reported cases of ectopic AOV over the line extending from the pylorus of the stomach down to the distal part of the duodenum. However, to the best of our knowledge, there are only five cases reported in the English literature of an ectopic AOV in the fourth part of the duodenum, with only one of them having adenocarcinoma of the ampulla. Hereby, we report the first case of ectopic AOV in the fourth part of the duodenum, presenting with obstructive due to adenocarcinoma with focal squamous differentiation. This is the case a 42-year-old lady who had a sleeve gastrectomy for morbid obesity in the past. She presented with right upper quadrant pain for one month associated with subjective fever, unintentional weight loss, pale stool, and dark urine. The physical examination revealed a deeply jaundiced lady with an unremarkable abdominal exam. A computed tomography scan of the abdomen revealed intrahepatic and extrahepatic biliary dilation with ectopic insertion of the distal CBD into the fourth part of the duodenum with no evidence of biliary stones. She underwent pancreaticoduodenectomy after difficult biliary decompression. Histopathological diagnosis was moderately differentiated adenocarcinoma, pancreaticobiliary type with focal squamous differentiation. Ectopic AOV is a very rare entity, especially when it is associated with adenosquamous carcinoma changes.
Introduction
Congenital anomalies of the gallbladder, biliary tree, and pancreas are rare; they can occur during any of the stages of embryogenesis. These congenital anomalies can present with a variety of symptoms, usually during infancy and childhood; hence, they are usually diagnosed early [1].
The main AOV, which is the convergence of the common bile duct (CBD) and pancreatic duct (PD), is typically located in the second part of the duodenum. An ectopic opening of the AOV into the third and fourth parts of the duodenum, stomach, and along the pyloric canal is very rare, with an incidence ranging from 5.6% to 23%. We can infer from this wide range that these anomalies can only be detected once symptomatic, explaining the scarcity of reported cases [2]. Some of these cases were presented as acute cholangitis, while others were discovered incidentally [2, 3].
However, to the best of our knowledge, there have been only five cases reported in the English literature of an ectopic AOV in the fourth part of the duodenum, with only one of them having adenocarcinoma of the ampulla. Herein, we report the first case of ectopic AOV in the 4th part of the duodenum, presenting with obstructive jaundice secondary to adenocarcinoma with focal squamous differentiation.
Case presentation
We report a case of a 42-year-old female medically free apart from sleeve gastrectomy in the past. She presented to our clinic complaining of right upper quadrant abdominal pain for 1 month. The pain was moderate, colicky, and associated with subjective fever, pale stool, dark urine, and unintentional weight loss.
On examination, she looked deeply jaundiced. Her abdominal exam was unremarkable. Her laboratory workup showed a picture of obstructive jaundice with elevated tumor markers, namely, CEA and Ca 19.9. An abdominal CT scan was done and showed diffused intrahepatic and extrahepatic biliary dilation with ectopic insertion of the distal CBD into the distal duodenum. However, no distal CBD stones were seen. But the bulging of the periampullary region, specifically at the upper border of the ampulla, nevertheless, no clearly delineated periampullary mass can be appreciated. Magnetic resonance cholangiopancreatography [MRCP] showed the same finding (Fig. 1).
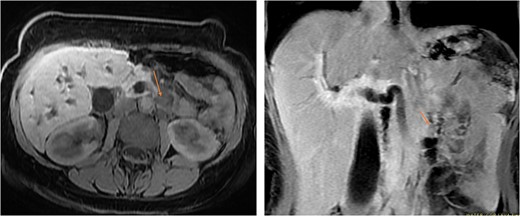
MRI in axial and sagittal views shows the location of the AOV tumor, as pointed out by the arrows.
Endoscopic ultrasound (EUS) was carried out to delineate the anatomy and the location of the ampulla of vater (AOV) and showed an abnormally located AOV in the distal duodenum (D4) with dilated CBD. Endoscopic retrograde cholangiopancreatography [ERCP] was performed in the same setting with difficulties in cannulating the ectopic AOV that was associated with mucosal abnormality. After multiple trials, the cannulated pancreaticogram was unremarkable (Fig. 2). The double-wire technique is used to cannulate CBD. Cholangiogram revealed a short distal filling defect suspicious for stones. Several balloon sweeps were done, but no stones were retrieved. Cytology brushing and fluoroscopically guided intraductal biopsy were done. Also, mucosal ampullary biopsies were taken as well. Then, a CBD stent was inserted. The cytology was inconclusive. However, the ampullary biopsy revealed high-grade dysplasia, and invasive carcinoma could not be excluded. Therefore, she was suspected to have ampullary malignancy.
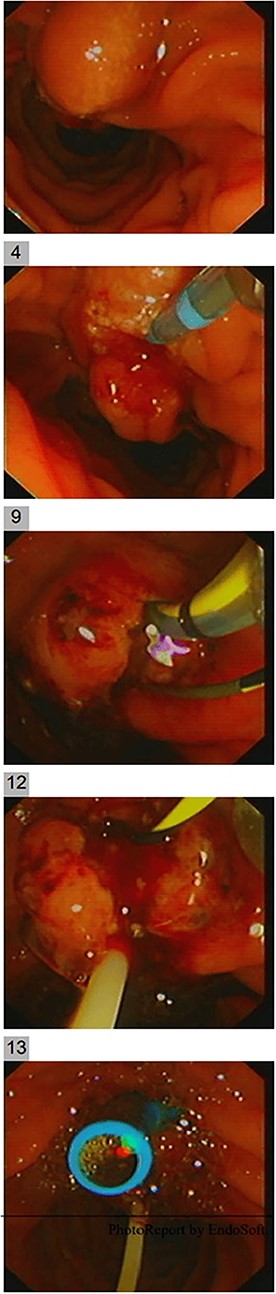
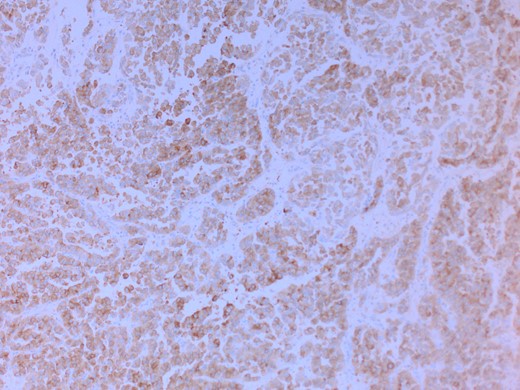
She had a CT chest to complete the staging workup, which was negative for metastasis. Eventually, she had a pancreaticoduodenectomy (Whipple procedure) with smooth postoperative recovery. The patient was discharged home on postoperative Day 7. The final histopathology came as moderately differentiated adenocarcinoma, pancreaticobiliary type with focal squamous differentiation (5%), invasion into the muscularis propria of the duodenum, and positive lymphovascular invasion with 1/12 LN positive for tumor deposits (Figs 3–6). All surgical margins were tumor-free. After a multidisciplinary meeting, the decision was made with proceed for adjuvant chemotherapy. Currently, she is on adjuvant chemotherapy, which she is tolerating well. Follow-up CT CAP at 3- and 6-month intervals postoperatively showed no evidence of recurrence or distant metastasis.
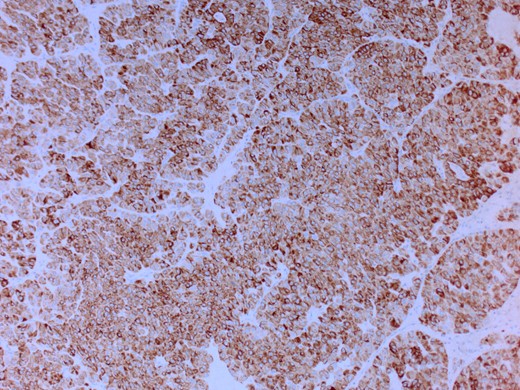
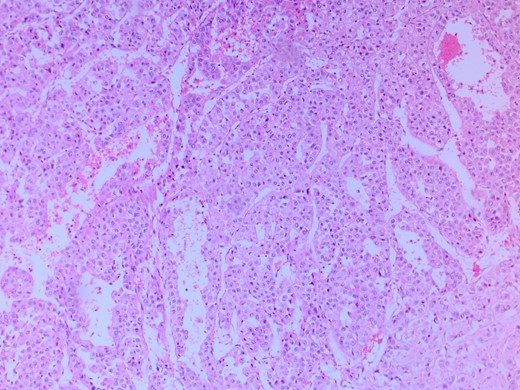
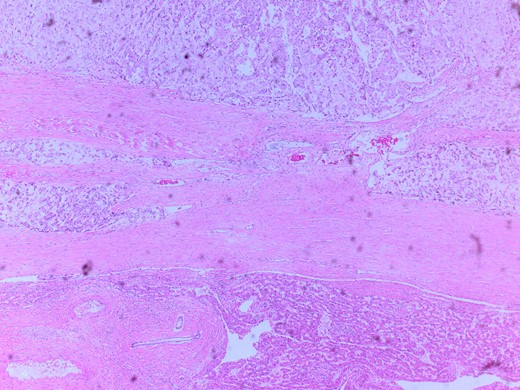
Microscopic image with H&E staining shows a tumor interface with normal liver parenchyma.
Discussion
The formation of an ectopic AOV has been correlated to the length of the common channel, i.e. the more distal the location of the papilla, the longer the common channel [4]. Thus, the formation of an ectopic AOV can be associated with multiple embryonic developmental disorders. Also, the location of the AOV is determined at the 22nd day of gestation, while the union of the pancreaticobiliary duct begins at the fifth week of gestation. Another theory suggests that the association between the anomalous pancreatic duct anatomy (PDA) and the ectopic distal location of the AOV is coupled with the presence of congenital bile duct dilatation (CBD) [5].
Adenosquamous carcinoma (ASC) of the AOV, which is a malignant tumor that exhibits both elements of the glandular and squamous tissue, has multiple theories of histogenesis; Yang et al. [6] have described four of them. The four theories are: (i) pluripotent stem cells that can induce the malignant transformation of both cell types, (ii) squamous metaplasia in the intestinal mucosa, (iii) malignant squamous metaplastic transformation of the adenocarcinoma, and (iv) collision of the two malignant tumors.
Hoshimoto et al. [7] have reported a summary of all the published cases of ACS and AOV in the English literature. Most of the cases presented with the following symptoms: biliary colic, jaundice, and constitutional symptoms [8]. Moreover, there was no sufficient evidence to establish a gender association. However, multiple case series and case reports have suggested that it is more common in males [8–10].
The diagnosis of ectopic AOV should be established by identifying the orifice in an anomalous location rather than the posteromedial part of the second duodenum. Multiple studies have reviewed the anomalous location of AOV. But Lee and Fu [9] summarized the most common locations of ectopic AOV, which were in the third or fourth part of the duodenum.
In a case series of 53 patients with an ectopic opening of AOV into the duodenal bulb done by Disibeyaz et al. [8], male patients were more common than females, with a ratio of 49:4. The patients were diagnosed with either acute cholangitis or recurrent stone disease. These patients underwent transabdominal ultrasonography as an initial study, followed by computed tomography (CT), and finally upper endoscopy with ERCP. In this study, the endoscopic approach was their primary diagnostic and therapeutic modality, and ERCP was performed successfully in 96% of the cases. However, the remaining 4% were diagnosed by a percutaneous transhepatic cholangiogram (PTC) due to failure of cannulation caused by the deformity of the duodenal bulb [8].
The management of symptomatic ectopic AOV can vary depending on the patient’s presentation and the underlying etiology. However, these patients can be managed surgically or endoscopically [11]. Regarding the complications, Balli et al. [12] presented that the utilization of ERCP in cases of bulbar ectopic AOV carries a statistically significant higher rate of pancreatitis, reaching up to 50% compared to only 16.7% for patients who have a normal location of AOV. Furthermore, the success rate of cannulation was insignificantly lower in cases of ectopic AOV in comparison to patients who have normally located AOV. As for ASC of the AOV, surgery remains the mainstay treatment for ASC of the AOV.
With regard to prognosis, the ASC of the AOV has a poor prognosis, with aggressive behavior and a survival rate reaching half that of pancreatic adenocarcinoma. It is speculated that the percentage of squamous cell differentiation within the tumor is related to its progression and aggressiveness, as demonstrated in animal models. Moreover, it appears that it has a high rate of recurrence, with two reported cases of death within 10 months after surgical intervention. Also, the overall survival, according to the summary of all seven reported cases of ASC and AOV in the English literature, ranged from 6 to 20 months [7].
Conclusion
The ectopic location of AOV is a very rare entity when it is associated with histopathological changes like ASC. Such patients usually present with jaundice and abdominal pain or other non-specific symptoms or even no symptoms. Biliary decompression is difficult, and preoperative histopathological diagnosis by biopsy is usually undermined. The diagnosis usually targets the staging and the extent of the tumor. The management mainly depends on the stage of the disease and the risk of surgical intervention. But the mainstay treatment is surgical resection of the ASC of the AOV at an early stage. The prognosis of the disease itself is unknown, but early detection and resection are recommended.
Author contributions
Study concept or design: A.S.Q., N.M.
Data collection: R.M.A., A.A.T.
Data interpretation: A.S.Q., M.A.S., N.M.
Literature review: R.M.A., M.A.S., A.S.Q.
Drafting of the paper: N.M., M.A.S.
Editing of the paper: M.A.S., A.S.Q.
Conflict of interest statement
None declared.
Consent
A written informed consent was obtained from the patient for publication.
References
- computed tomography
- weight reduction
- adenocarcinoma
- fever
- adenosquamous carcinoma
- feces
- jaundice
- pallor
- pancreaticoduodenectomy
- vater's ampulla
- diagnosis
- duodenum
- pylorus
- stomach
- urine
- abdominal examination
- sleeve gastrectomy, laparoscopic
- common bile duct decompression
- surgical dilation of bile duct
- histopathology tests



