-
PDF
- Split View
-
Views
-
Cite
Cite
Hafsa El ouazzani, Olaya Hamidi, Alain Habimana, Bouchra Dani, M Boulaadas, Fouad Zouaidia, Nadia Cherradi, Primary epithelioid angiosarcoma of the mandibular gingiva: diagnostic pitfalls, about an unusual entity, Journal of Surgical Case Reports, Volume 2024, Issue 5, May 2024, rjae323, https://doi.org/10.1093/jscr/rjae323
Close - Share Icon Share
Abstract
Primary angiosarcoma of the oral cavity is a rare malignant vascular neoplasm variably recapitulating endothelial cells and is generally associated with a worse prognosis. The epithelioid subtype is even uncommon in this localization. To our knowledge, only seven cases of primary oral epithelioid angiosarcoma have been reported until 2021. This histopathological variant is characterized by solid and sheet-like growth patterns that may be misinterpreted as other lesions with epithelioid cells. Herein, we present a new case of primary epithelioid angiosarcoma of the mandibular gingiva to discuss histopathological differential diagnoses and potential diagnostic pitfalls.
Introduction
Angiosarcoma is a malignant vascular neoplasm variably recapitulating endothelial cells [1]. In the oral cavity, it represents ≈1% of all angiosarcomas [1, 2] and occurs preferentially in males with a high incidence in the elderly [1].
Commonly, this sarcoma manifests an aggressive clinical course and a poor prognosis [3]. However, the prognosis is more favorable for lip and tongue primaries but metastasis and recurrences are common [1]. Histologically, this tumor shows a large spectrum of morphological patterns; the epithelioid subtype [epithelioid angiosarcoma (EA)] is even rarer and is characterized by solid and sheet-like growth patterns. Its frequent immunohistochemical expression of epithelial markers can lead to misdiagnosis of carcinoma [4].
Case
We present the case of a 73-year-old man with no significant previous medical history. He experienced purple swelling in the right mandibular gingiva (Fig. 1a). The clinical examination was normal. However, a panoramic radiograph revealed bone resorption around the crowns of the 44 and 46 teeth (Fig. 1b). Further examination, including an extraoral examination and TAP CT scan, did not reveal any other mass or lymphadenopathy.
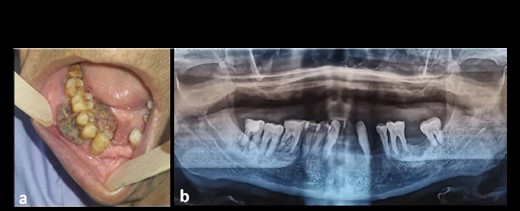
(a) Clinical image of an intraoral EA localized at the mandibular gingiva with extensive red-purple appearance; (b) orthopantomographic image showing resorption of the mandibular bone around the crown of the 44 and 46 teeth.
During the histopathological analysis of the biopsy, it was found that the tumor consisted of solid sheets with incomplete alveolar and vasoformative structures. The tumor cells showed abundant amphophilic cytoplasm, large vesicular nuclei, and prominent nucleoli. Mitotic figures were scattered throughout the tumor (Fig. 2a–c). Immunohistochemical analysis revealed positivity for vascular markers (CD31 and transcriptional regulator ERG) and epithelial markers (CKAE1/AE3, CK7), and negativity for CD34, basaloid markers (P40, CK5/6), and melanic markers (MelanA, HMB45) (Fig. 2d–g). The ki67 index was estimated at 50%. These features pointed to a diagnosis of EA. Initially, the patient underwent a local surgical excision and was recommended for palliative radiochemotherapy. After 2 months, the tumor recurred, with an extension to the mandibular bone. As a result, the patient underwent a segmental mandibulectomy, but he died postoperatively.
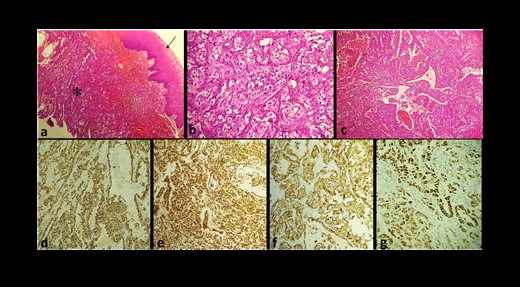
(a) Microphotography of EA showing a solid and sheet-like proliferation (star) under the squamous epithelium covering the gingival mucosa (arrow) (Hematoxylin–Eosin ×10); tumor cells show marked cellular pleomorphism with abundant eosinophilic cytoplasm (Hematoxylin–Eosin ×10); (c) the tumor exhibits a vasoformative architecture; at IHC, tumor cells express CD31 (d), ERG (e), CKAE1/AE3 (f), and CK7 (g).
Discussion
Angiosarcoma is a malignant tumor that recapitulates the morphological and functional features of the endothelium to a variable degree [5]. It arises most commonly in the deep muscles of the lower extremities, retroperitoneum, mediastinum, and mesentery [5]. Primary angiosarcomas in the oral cavity represent only 1% of all angiosarcomas [1, 2]; the epithelioid variant is even rarer in this localization [6]. To our knowledge and based on the English-language literature, only seven cases of primary oral EA have been reported. Four of them were described in the review of Yuko Komatsu et al. [6]. In this paper, we summarize the clinical features, treatment, follow-up, and immunophenotype of three others, together with our case in Table 1 [3, 4, 6–10]. Upon analysis of these cases, it was observed that EA is more frequently found in older men, with a preference for maxillary localization. Mandibular origin was observed in two cases. Almost all cases presented recurrence and metastasis.
| . | Author/year . | Age . | Sex . | Primary site . | Treatment . | Follow Up . | Vascular markers . | Epithelial markers . |
|---|---|---|---|---|---|---|---|---|
| 1 | Paul D Freedman et al./1992 [7] | 32 Y | M | Maxilla (hard palate) | Resection of left side of maxilla | No recurrence No metastasis (For 18 months) | Factor VIII + | NA |
| 2 | Masaru Sasaki et al./1996 [4] | 69 y | M | Maxilla (hard palate) | Partial maxillectomy + radio-Chemotherapy | Metastasis in stomach, cerebrum, and 12th thoracic vertebra | CD31 + Factor VIII + | Cytokeratin+ EMA- |
| 3 | Gianfranco Favia et al./2002 [8] | 74 y | F | Maxilla (hard palate) | Partial maxillectomy+ chemotherapy | Died in 3 months | CD34- CD31 + Factor VIII - | CKAE1/AE3+ |
| 4 | Triantafillidou K et al./2002 [10] | 50 y | F | Left maxilla | Left medial maxillectomy | No recurrence No metastasis (for 3 months) | Factor VIII + | NA |
| 5 | Abbas Agaimy/ 2012 [9] | 71 y | F | Palatine tonsil | Surgical resection | Intra oral and gastro-intestinal metastasis | CD34 + CD31 + Factor VIII + ERG + | CKAE1/AE3+ |
| 6 | Nagata et al./ 2014 [3] | 55 y | M | Mandibular gingiva | Segmental mandibular resection | Thoracic and vertebral metastasis | CD34 + | EMA + CKAE1/AE3 - |
| 7 | Yoko Komatsu et al./2020 [6] | 66 y | M | Mandibular gingiva | Segmental mandibular resection | No recurrence No metastasis (for 2 years) | CD31 + Factor VIII + | Cytokeratin- |
| 8 | Hafsa El Ouazzani et al./2023 | 73 y | M | Mandibular gingiva | Local surgical excision followed by segmental mandibulectomy | Recurrence and died in 2 months | CD31 + CD34 - ERG + | CKAE1/AE3+ CK7+ EMA - |
| . | Author/year . | Age . | Sex . | Primary site . | Treatment . | Follow Up . | Vascular markers . | Epithelial markers . |
|---|---|---|---|---|---|---|---|---|
| 1 | Paul D Freedman et al./1992 [7] | 32 Y | M | Maxilla (hard palate) | Resection of left side of maxilla | No recurrence No metastasis (For 18 months) | Factor VIII + | NA |
| 2 | Masaru Sasaki et al./1996 [4] | 69 y | M | Maxilla (hard palate) | Partial maxillectomy + radio-Chemotherapy | Metastasis in stomach, cerebrum, and 12th thoracic vertebra | CD31 + Factor VIII + | Cytokeratin+ EMA- |
| 3 | Gianfranco Favia et al./2002 [8] | 74 y | F | Maxilla (hard palate) | Partial maxillectomy+ chemotherapy | Died in 3 months | CD34- CD31 + Factor VIII - | CKAE1/AE3+ |
| 4 | Triantafillidou K et al./2002 [10] | 50 y | F | Left maxilla | Left medial maxillectomy | No recurrence No metastasis (for 3 months) | Factor VIII + | NA |
| 5 | Abbas Agaimy/ 2012 [9] | 71 y | F | Palatine tonsil | Surgical resection | Intra oral and gastro-intestinal metastasis | CD34 + CD31 + Factor VIII + ERG + | CKAE1/AE3+ |
| 6 | Nagata et al./ 2014 [3] | 55 y | M | Mandibular gingiva | Segmental mandibular resection | Thoracic and vertebral metastasis | CD34 + | EMA + CKAE1/AE3 - |
| 7 | Yoko Komatsu et al./2020 [6] | 66 y | M | Mandibular gingiva | Segmental mandibular resection | No recurrence No metastasis (for 2 years) | CD31 + Factor VIII + | Cytokeratin- |
| 8 | Hafsa El Ouazzani et al./2023 | 73 y | M | Mandibular gingiva | Local surgical excision followed by segmental mandibulectomy | Recurrence and died in 2 months | CD31 + CD34 - ERG + | CKAE1/AE3+ CK7+ EMA - |
y: year; M: male; F: female; NA: not available.
| . | Author/year . | Age . | Sex . | Primary site . | Treatment . | Follow Up . | Vascular markers . | Epithelial markers . |
|---|---|---|---|---|---|---|---|---|
| 1 | Paul D Freedman et al./1992 [7] | 32 Y | M | Maxilla (hard palate) | Resection of left side of maxilla | No recurrence No metastasis (For 18 months) | Factor VIII + | NA |
| 2 | Masaru Sasaki et al./1996 [4] | 69 y | M | Maxilla (hard palate) | Partial maxillectomy + radio-Chemotherapy | Metastasis in stomach, cerebrum, and 12th thoracic vertebra | CD31 + Factor VIII + | Cytokeratin+ EMA- |
| 3 | Gianfranco Favia et al./2002 [8] | 74 y | F | Maxilla (hard palate) | Partial maxillectomy+ chemotherapy | Died in 3 months | CD34- CD31 + Factor VIII - | CKAE1/AE3+ |
| 4 | Triantafillidou K et al./2002 [10] | 50 y | F | Left maxilla | Left medial maxillectomy | No recurrence No metastasis (for 3 months) | Factor VIII + | NA |
| 5 | Abbas Agaimy/ 2012 [9] | 71 y | F | Palatine tonsil | Surgical resection | Intra oral and gastro-intestinal metastasis | CD34 + CD31 + Factor VIII + ERG + | CKAE1/AE3+ |
| 6 | Nagata et al./ 2014 [3] | 55 y | M | Mandibular gingiva | Segmental mandibular resection | Thoracic and vertebral metastasis | CD34 + | EMA + CKAE1/AE3 - |
| 7 | Yoko Komatsu et al./2020 [6] | 66 y | M | Mandibular gingiva | Segmental mandibular resection | No recurrence No metastasis (for 2 years) | CD31 + Factor VIII + | Cytokeratin- |
| 8 | Hafsa El Ouazzani et al./2023 | 73 y | M | Mandibular gingiva | Local surgical excision followed by segmental mandibulectomy | Recurrence and died in 2 months | CD31 + CD34 - ERG + | CKAE1/AE3+ CK7+ EMA - |
| . | Author/year . | Age . | Sex . | Primary site . | Treatment . | Follow Up . | Vascular markers . | Epithelial markers . |
|---|---|---|---|---|---|---|---|---|
| 1 | Paul D Freedman et al./1992 [7] | 32 Y | M | Maxilla (hard palate) | Resection of left side of maxilla | No recurrence No metastasis (For 18 months) | Factor VIII + | NA |
| 2 | Masaru Sasaki et al./1996 [4] | 69 y | M | Maxilla (hard palate) | Partial maxillectomy + radio-Chemotherapy | Metastasis in stomach, cerebrum, and 12th thoracic vertebra | CD31 + Factor VIII + | Cytokeratin+ EMA- |
| 3 | Gianfranco Favia et al./2002 [8] | 74 y | F | Maxilla (hard palate) | Partial maxillectomy+ chemotherapy | Died in 3 months | CD34- CD31 + Factor VIII - | CKAE1/AE3+ |
| 4 | Triantafillidou K et al./2002 [10] | 50 y | F | Left maxilla | Left medial maxillectomy | No recurrence No metastasis (for 3 months) | Factor VIII + | NA |
| 5 | Abbas Agaimy/ 2012 [9] | 71 y | F | Palatine tonsil | Surgical resection | Intra oral and gastro-intestinal metastasis | CD34 + CD31 + Factor VIII + ERG + | CKAE1/AE3+ |
| 6 | Nagata et al./ 2014 [3] | 55 y | M | Mandibular gingiva | Segmental mandibular resection | Thoracic and vertebral metastasis | CD34 + | EMA + CKAE1/AE3 - |
| 7 | Yoko Komatsu et al./2020 [6] | 66 y | M | Mandibular gingiva | Segmental mandibular resection | No recurrence No metastasis (for 2 years) | CD31 + Factor VIII + | Cytokeratin- |
| 8 | Hafsa El Ouazzani et al./2023 | 73 y | M | Mandibular gingiva | Local surgical excision followed by segmental mandibulectomy | Recurrence and died in 2 months | CD31 + CD34 - ERG + | CKAE1/AE3+ CK7+ EMA - |
y: year; M: male; F: female; NA: not available.
In our experience as a pathologist, diagnosing oral EA may be a challenge. Grossly, EA shows a bloody cut surface with a red to blush-purple appearance [1], sometimes resembling a pyogenic granuloma. Histologically, it is characterized by sheet-like, atypical multilayered, or solid endothelial proliferation. It displays a greater degree of nuclear pleomorphism and mitotic activity and frequently shows areas of necrosis [6]. Vasoformative architecture with intracytoplasmic vacuoles (neolumen) containing erythrocytes is useful for the diagnosis of EA [1], However, immunohistochemistry (IHC) remains the gold standard for the definitive diagnosis. EA generally shows positivity for CD31, CD34, and ERG. Although CD31 and CD34 can show variable expression [11], ERG has the highest sensitivity (reaching 100%) and specificity for identifying vascular and endothelial differentiation [11].
EA is an exceedingly rare variant and closely mimics spindle or acantholytic cells squamous cell carcinoma [8], causing great diagnosis confusion, particularly if the tumor also expresses the epithelial markers such as cytokeratins and epithelial membrane antigen (EMA) [9]. Miettinen et al. [12] found in their study that 50% of EA stained with CK8 and CK18 but less commonly with CK7 and CK19. Interestingly, high-molecular-weight cytokeratins (CK14 and 34βE12) have not been detected in vascular neoplasms [9] unlike squamous cell carcinoma.
On the other hand, EA may be confused with other malignant lesions presenting with epithelioid cells such as epithelioid sarcoma or melanoma [11]. In practice, the negativity of melanic markers (MelanA and HMB45) excludes melanoma, while the positivity of ERG and CD 31 excludes carcinoma and epithelioid sarcoma [11].
EA must also be distinguished from other vascular tumors with epithelioid morphology such as epithelioid hemangioma (EH) and epithelioid hemangioendothelioma (EHE). EH is a benign tumor that lacks pleomorphism and destructive growth [1]. At the same time, EA is a high-grade malignancy characterized by an infiltrative growth pattern. It displays a greater degree of nuclear pleomorphism and mitotic activity than EHE and frequently shows areas of necrosis [10]. It is a very destructive tumor, has a propensity to recur locally, spread extensively, and has an elevated rate of lymph node and systemic metastases, with tumor-related death [13].
Conclusion
To summarize, it is important to consider various possible diagnoses before concluding that a patient has primary oral EA. Furthermore, it is crucial to rule out any possibility of secondary metastatic disease before making a final diagnosis. We recommend that prompt and accurate diagnosis can lead to a better prognosis for this type of tumor.
Conflict of interest statement
The authors declare that they have no competing interests.
Funding
No external funding sources are relevant to this submission.
Data availability
No new data were generated or analyzed in support of this research.
Consent for publication
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patient’s legally authorized.



