-
PDF
- Split View
-
Views
-
Cite
Cite
Kirsten R Carlaw, Chandika Liyanage, A rare case of perforated Meckle’s diverticulitis: a case report, Journal of Surgical Case Reports, Volume 2024, Issue 5, May 2024, rjae315, https://doi.org/10.1093/jscr/rjae315
Close - Share Icon Share
Abstract
Meckle’s diverticulum is the most common embryological anomaly of the small bowel that is rarely seen in adults. It is caused by the incomplete closure of the vitelline or omphalomesenteric duct. Those who are symptomatic from Meckle’s diverticulum have varied clinical presentations, which raise significant challenges with diagnostic and management options. We report a case of a 47-year-old male who presented to the hospital with clinical signs of appendicitis but was found to have perforated Meckle’s diverticulitis with faecoliths on computed tomography imaging and laparoscopy. Furthermore, histopathology revealed an ectopic gastric tissue cell type, which is a rare finding. This was definitively managed surgically with laparoscopic resection of Meckle’s diverticulum and appendicectomy.
Introduction
Meckle’s diverticulum is a true small bowel diverticulum due to the persistence of the omphalomesenteric duct [1]. It is the most common congenital anomaly of the gastrointestinal tract affecting 2%–4% of people [1]. The majority are asymptomatic, although complications include haemorrhage, intestinal obstruction, intussusception, perforation, and vesicodiverticular fistulae [2]. Furthermore, symptomatic patients are predominantly children, and it is rare to encounter this in adults [3]. This raises potential diagnostic dilemmas and management issues.
Our aim is to discuss a rare case of perforated Meckle’s diverticulitis that was initially presumed to be clinical appendicitis on history and examination but, on further assessment with computed tomography imaging and laparoscopy, was deemed to be a complication of Meckle’s diverticulum.
Case report
A 47-year-old male presented with 2–3 days of worsening constant, migratory lower abdominal pain that localized to the right lower quadrant 12 hr prior to review. The pain was exacerbated with movement. He reported subjective fevers, nausea, and an episode of bilious vomiting. He had normal bowel motions that morning and denied lower urinary tract symptoms. He denied previous episodes of abdominal pain.
Relevant past medical history included a previous lithotripsy and ureteric stent for renal calculi, an open right inguinal hernia repair with mesh, and scrotal exploration for testicular torsion as a child. He denied any medical conditions or bowel disease. He did not take regular medications and denied any previous gastroscopies or colonoscopies. His mother had bowel cancer when she was around 60 years old.
On examination, he was febrile to 38°C, had sinus tachycardia to 110, respiratory rate and blood pressure were within the normal range. His abdomen was soft, non-distended, and tender in the lower abdomen with localized peritonism in the right lower quadrant. No clinical hernias were detected.
Blood tests demonstrated significantly raised inflammatory markers, including white blood cell count 20 × 10^9/l and C-reactive protein of 68 mg/l. Other parameters, including liver function test, and urea and electrolytes analyses were all within normal range. The urine analysis was unremarkable. Our initial clinical diagnosis was acute appendicitis.
The computerized tomography of abdomen and pelvis (CTAP) with intravenous contrast scan (Fig. 1a–c) revealed a distended, fluid-filled blind-ending structure, containing calcified bodies, arising from the small bowel within the mid-lower abdomen with adjacent inflammatory changes. This reflected a likely case of Meckle’s diverticulitis. The appendix was normal, and the remaining abdominal viscera was unremarkable. No lymphadenopathy was evident, and the vascular structures were unremarkable. There was no abdominal fluid or gas.
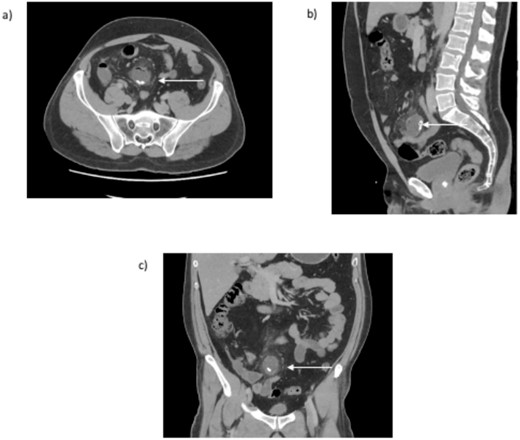
CT abdomen and pelvis (a) transversal, (b) sagittal, and (c) coronal view. An arrow indicates the inflamed blind ending structure of the small bowel with calcified bodies.
Initial preoperative management included intravenous (IV) augmentin and crystalloid fluid, analgesia, and antiemetics. The patient underwent an operation the same day via laparoscopic approach with two 12 mm ports and one 5 mm port. There was purulent fluid in the right iliac fossa with a normal appendix but matted ileal loops in the right iliac fossa revealing a Meckle’s diverticulum with gangrenous, perforated tip, and a base of 1 cm in width. The diverticulum was dissected from bowel adhesions, base identified, and resected with a linear echelon blue stapler (Fig. 2a and b). Appendicectomy was also performed. Both specimens were sent for histology. A thorough wash and meticulous haemostasis were performed. A 19Fr Blake drain was left in the pelvis.
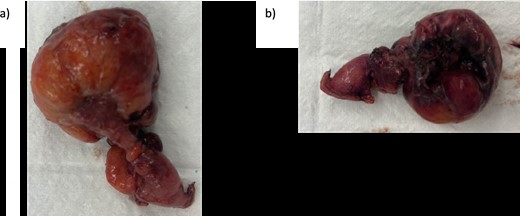
Intraoperative specimen (a) anterior view and (b) posterior view of resected Meckle’s diverticulum.
Postoperatively, the patient progressed well, remained afebrile, and was hemodynamically stable, with significantly improved inflammatory markers. The pelvic drain had minimal haemoserous outputs and was removed 3 days postoperatively. He was discharged 5 days postoperatively having completed 5 days of IV Augmentin.
Histopathology revealed a normal appendix with no significant acute inflammatory cell infiltrate. Sections of the Meckel’s diverticulum showed small intestinal wall with haemorrhage, mucosal necrosis, area of gangrenous change, and associated acute inflammatory cell infiltrates with suppuration and probably secondary vasculitis. Ischemic changes are noted. Ectopic gastric tissue with oxyntic-type glands and parietal cells was focally noted. No evidence of malignancy was seen. Meckle’s diverticulum measured 28 mm × 30 mm × 20 mm and contained a large cystic cavity with faecal material.
Discussion
Meckle’s diverticulum is rare in the adult population and is formed due to incomplete obliteration of the vitelline duct [2]. It is typically located 2 feet from the ileocolic junction, is 2 inches in length, and is lined with normal small bowel mucosa [3]. Other cell types, including ectopic gastric (20%–57% of cases), duodenal, colonic, pancreatic, and endometrial mucosa, may also be present [4]. In our case, ectopic gastric tissue was noted, which can result in acid production and local inflammatory complications, such as significant ulceration and perforation [4].
Symptomatic Meckle’s diverticulum typically occurs in children aged 2–8 years [2]. In adults, frequent complications include intestinal obstruction due to intussusception or adhesive band (14%–53%), ulceration (<4%), diverticulitis, or rarely perforation or bleeding [5]. Our patient had a perforated Meckle’s diverticulitis.
Preoperative diagnosis of symptomatic Meckle’s diverticulum is challenging (11% of cases) [6]. Technetium-99m pertechnate scan is commonly used due to the increased uptake in the ectopic gastric mucosa allowing visualization of the Meckle’s diverticulum [7]. However, it has a sensitivity of 62.5% and specificity of 9% in adult patients due to false positives in gut duplication cysts and other ectopic gastric mucosa [8]. A CTAP scan may show the diverticulum and signs of inflammation or obstruction, as was evident with our patient. Other investigations include mesenteric arteriography if presenting with intestinal bleeding, double-balloon enteroscopy, and capsule endoscopy [9]. If diagnostic testing is inconclusive or the patient is haemodynamically unstable, operative intervention with diagnostic laparoscopy or laparotomy is indicated [10].
Recent data demonstrates that preferential management for symptomatic Meckle’s diverticulum is laparoscopic resection rather than open approach due to improved complication risk, cost-effectiveness, recovery time, and length of hospital stay [11]. Diverticulectomy with transverse ileal closure is recommended to minimize the risk of stenosis; in our case, this was performed with linear stapler across the diverticulum base [12]. Surgical resection for asymptomatic Meckle’s diverticulum is controversial, although there is a growing trend for prophylactic diverticulectomy, particularly with the characteristics of a longer diverticulum (>2 cm), males <40–50 years old, and presence of histologically abnormal tissue [13]. Studies have shown a 0%–6% risk of significant postoperative morbidity following incidental resection versus 33% after resection of symptomatic or complicated Meckle’s diverticulum [13].
Conclusion
Meckle’s diverticulitis in adults is a very rare and often difficult clinical diagnosis that can present like acute appendicitis. Investigation with CTAP is useful, and laparoscopic resection is the preferred treatment. The presence of ectopic gastric tissue predisposes to significant ulceration and a high risk of perforation.
Conflict of interest statement
None declared.
Funding
None declared.



