-
PDF
- Split View
-
Views
-
Cite
Cite
Andrej Nikolovski, Nimetula Limani, Aleksandra Ristova Tancheva, Antonela Manasievska Bogoevska, Blagica Krsteska, Small intestine metastasis from endometrial carcinoma initially presented as enterocutaneous fistula: a case report and literature review, Journal of Surgical Case Reports, Volume 2024, Issue 5, May 2024, rjae297, https://doi.org/10.1093/jscr/rjae297
Close - Share Icon Share
Abstract
Although endometrial cancer is the fourth most common malignancy among women, it rarely metastasizes to the small intestine. Cases of endometrial recurrence to the intestine clinically present with secondary anemia, melena, abdominal cramps, and epigastric pain. Only a dozen cases are reported in the literature, but none presented with an enterocutaneous fistula. In this report, we present a case of an 88-year-old female patient previously treated for endometrial adenocarcinoma with surgery and adjuvant radiotherapy. Fourteen months after the surgery, the patient presented with an enterocutaneous fistula on the anterior abdominal wall, which was confirmed to be a metastasis from the primary tumor. To our knowledge, this is the first case of endometrial cancer metastasizing to the small intestine with involvement of the anterior abdominal wall and the occurrence of an enterocutaneous fistula, which was treated with radical surgery.
Introduction
Regardless of the proper treatment of primary endometrial cancer (EC) depending on its pretreatment stage, the risk for local and distant spread remains. Recurrent EC is typically seen in the pelvic and paraaortic lymph nodes, the peritoneum (peritoneal carcinomatosis), and the lungs. It rarely spreads to the extra abdominal lymph nodes, the musculoskeletal and nervous system, and the intraabdominal organs [1]. Isolated metastases to the small intestine are extremely rare [1, 2]. Depending on the small intestine affection level, the metastasis may infiltrate the abdominal wall and its skin thus resulting in enterocutaneous fistula formation. Such ‘complicated’ small intestine metastasis from EC is shown in this report. To our knowledge, this is the first case of EC small intestine metastasis initially presented as an enterocutaneous fistula. Written informed consent was obtained from the patient for this case report.
Case report
An 88-year-old female patient was admitted to our clinic due to abdominal wall skin inflammation characterized by a visible fistulous opening spontaneously discharging enteric fluid, along with a palpable tumor (Fig. 1). The fistula presented as low output with <200 ml/24 h enteral discharge. The symptoms lasted for 1 month, and the patient was treated in the outpatient ward as having a cutaneous infection.
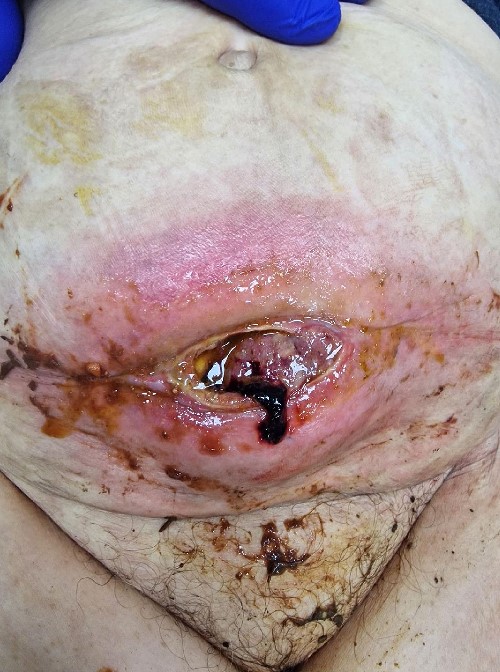
Clinical presentation of the metastasis as enterocutaneous fistula.
The patient’s history revealed previous treatment (14 months earlier) for mixed serous and endometrioid adenocarcinoma of the endometrium. The adenocarcinoma itself was with Grade 3, with present lymphovascular and cervical stromal invasion, R0 resection margins, and N0 nodal status. According to the International Federation of Gynecology and Obstetrics, the tumor was in postoperative Stage II. The treatment consisted of an abdominal hysterectomy with bilateral salpingo-oophorectomy and an adjuvant radiotherapy (external beam radiation therapy and high-dose-rate brachytherapy boost).
On the present admission, serum analysis showed a hemoglobin value of 107.00 g/l (120.0–165.0 g/l), hematocrit value of 0.32 I/l (0.35–0.5 I/l), serum glucose value of 6.90 mmol/l (3.9–5.83 mmol/l), C-reactive protein level of 108.10 mg/l (0.0–5.0 mg/l), serum total proteins value of 61.30 g/l (64.0–83.0 g/l), and serum albumin level of 26.90 g/l (35.0–50.0 g/l). The overall nutritional status of the patient was within the normal range.
A contrast-enhanced abdominal computerized tomography (CT) revealed the existence of a soft-tissue irregularly contoured tumor originating from the small intestine that breaks through the anterior abdominal wall and penetrates and infiltrates the subcutaneous fat as well as the skin. The tumor was measured to have dimensions of 89 × 75 mm (Fig. 2). The patient was offered a surgical exploration. A midline laparotomy beginning above the umbilicus was performed and when extending it to the caudal, en-block resection of the anterior abdominal wall was performed along with the affected small intestinal loop (the mid-portion of the ileum) containing the tumor (Figs 3–5). Primary end-to-end anastomosis was created. The abdominal wall defect was closed with a primary suture with a relative tension present on the suture line. The postoperative hospital period was uneventful. The patient was discharged on postoperative day 9. After discharge, a superficial surgical site wound infection occurred and was treated in the outpatient ward.
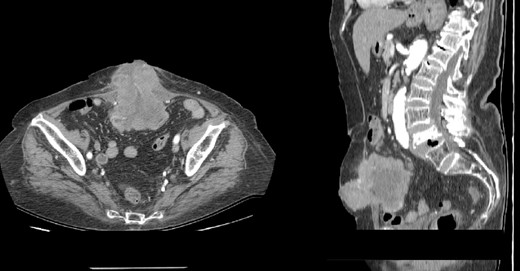
(A–B) Contrast-enhanced abdominal CT scan showing intraabdominal tumor that infiltrates the abdominal wall and the surrounding skin (A-axial scan, B-sagittal scan).
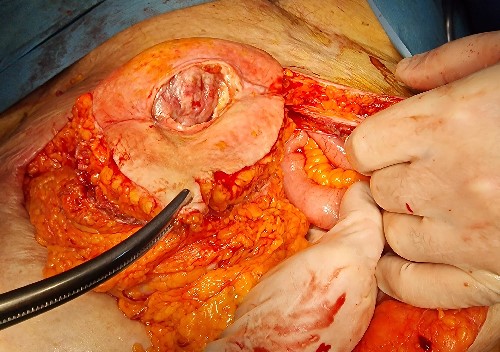
Caudal extension of the laparotomy with partial resection of the abdominal wall along with the enterocutaneous fistula.
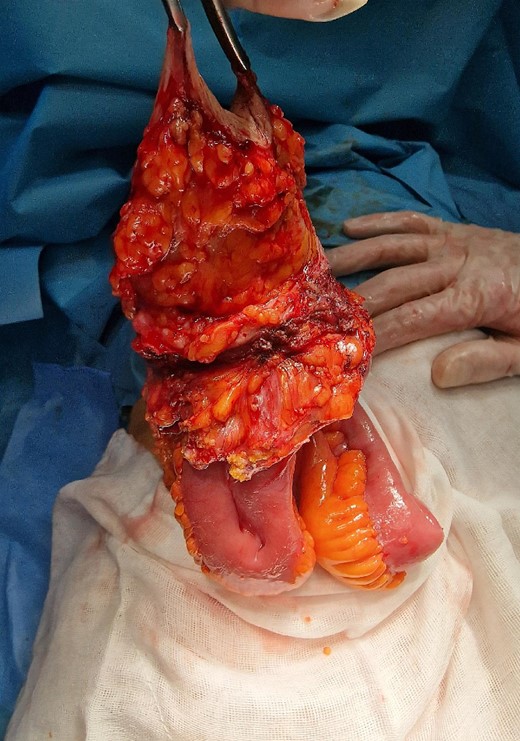
The operative specimen before detachment (resection of the small intestine).
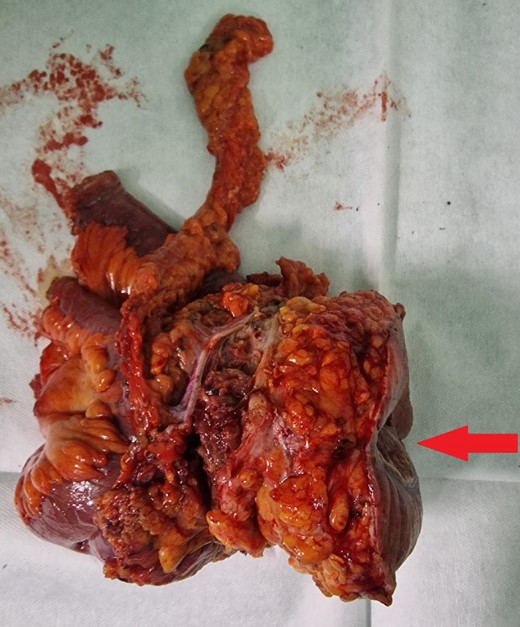
Resected specimen containing part of the small intestine with metastasis, part of the abdominal wall, and the skin with enterocutaneous fistula (arrow).
The macroscopic pathology report revealed the presence of an enterocutaneous fistula with an opening of 4.2 × 2.4 cm with tumor tissue emerging from within. The small intestine contained ulcerous neoplasm infiltrating its wall, the abdominal wall muscles, the subcutaneous fat, and the abdominal wall skin. The histology showed an infiltrative neoplasm that ulcerates the small intestinal mucosa and diffusely spread to the adherent abdominal muscle, resulting in the formation of an enterocutaneous fistula. Neoplastic cells were negative for Caudal-type homeobox 2 by immunohistochemical analysis and showed positive staining for Cytokeratin 7 (CK7) and Paired box gene 8 (PAX8), which favors endometrial origin (Fig. 6A–D). Considering the patient’s history of the previous malignant condition, the final diagnosis was a metastasis from EC to the small intestine with a subsequent enterocutaneous fistula.
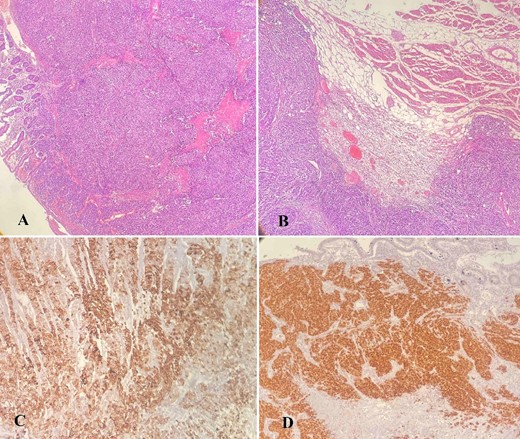
(A–D) Histology analysis; (A) tumor cells infiltrating the wall of the small intestine with mucosal ulceration (hematoxylin–eosin stain, ×40); (B) neoplastic cells infiltrating skeletal muscle of the abdominal wall (hematoxylin–eosin stain, ×40); (C) positive staining for CK7, ×100; (D) positive staining for PAX8, ×100.
Following surgery, the patient was referred to an oncologist where a plan for first-line adjuvant treatment was commenced.
Discussion
Even though EC is the fourth most common malignancy among women, intraabdominal metastases are a rare occurrence [3]. The liver is the most commonly affected intraabdominal organ (7%), followed by the adrenal glands and spleen (1%) [2]. Small intestine metastasis occurrence from EC is sporadic [1, 2, 4]. The current case is the 13th report of EC metastasis to the small intestine (including the duodenum). However, none of the previously reported cases presented clinically with an enterocutaneous fistula (Table 1).
| Reference number . | Author/year . | Patient age (years) . | Tumor stage . | Timing of metastasis presentation . | Clinical presentation . | Treatment . |
|---|---|---|---|---|---|---|
| [5] | Bosscher et al./1994 | 55 | IV B | Diagnosed during index surgery | – | Small intestine partial resection |
| [6] | Kirk et al./1999 | 64 | Not reported | 10 months after index surgery | Intermittent abdominal cramps and melena | Small intestine partial resection |
| [8] | Thijs et al./2007 | 85 | Not reported | 2 years after index surgery | Anemia | Small intestine partial resection |
| [10] | Tsai et al./2007 | 67 | Early-stage | 8 years after index surgery | Epigastric pain, melena | Segmental duodenectomy with Roux-en-Y duodenojejunostomy |
| [11] | Gallotta et al./2015 | 58 | I B | 13 months after index surgery | Not reported | Laparoscopic small intestine resection |
| [9] | Hubers and Soni/2017 | 75 | I B | 3 years after index surgery | Blood per rectum (synchronous sigmoid colon metastasis) | Small intestine partial resection + low anterior rectal resection |
| [7] | Leitão et al./2017 | 72 | Not reported | 5 years after index surgery | Melena | Palliative chemotherapy |
| [2] | Makki et al./2019 | 88 | Not reported | 5 months after index surgery | Anemia, abdominal pain | Small intestine partial resection |
| [12] | Huynh et al./2019 | Not reported | Not reported | 3 years after index surgery | Right lower back pain | Duodenal resection |
| [3] | Singh et al./2019 | 77 | I A | 3.5 years after index surgery | Melena, anemia | Gastroduodenal artery embolization + radiation therapy |
| [13] | Emiloju et al./2020 | 60 | Not reported | 10 months after index surgery | Abdominal pain, melena, generalized weakness | Pancreaticoduodenectomy |
| [4] | Jang et al./2022 | 55 | I B | 6 months after index surgery | Recurrent epigastric pain | Small intestine partial resection |
| Current case | Nikolovski et al./2024 | 88 | II | 14 months after index surgery | Entero-cutaneous fistula | En-block resection of small intestine with abdominal wall |
| Reference number . | Author/year . | Patient age (years) . | Tumor stage . | Timing of metastasis presentation . | Clinical presentation . | Treatment . |
|---|---|---|---|---|---|---|
| [5] | Bosscher et al./1994 | 55 | IV B | Diagnosed during index surgery | – | Small intestine partial resection |
| [6] | Kirk et al./1999 | 64 | Not reported | 10 months after index surgery | Intermittent abdominal cramps and melena | Small intestine partial resection |
| [8] | Thijs et al./2007 | 85 | Not reported | 2 years after index surgery | Anemia | Small intestine partial resection |
| [10] | Tsai et al./2007 | 67 | Early-stage | 8 years after index surgery | Epigastric pain, melena | Segmental duodenectomy with Roux-en-Y duodenojejunostomy |
| [11] | Gallotta et al./2015 | 58 | I B | 13 months after index surgery | Not reported | Laparoscopic small intestine resection |
| [9] | Hubers and Soni/2017 | 75 | I B | 3 years after index surgery | Blood per rectum (synchronous sigmoid colon metastasis) | Small intestine partial resection + low anterior rectal resection |
| [7] | Leitão et al./2017 | 72 | Not reported | 5 years after index surgery | Melena | Palliative chemotherapy |
| [2] | Makki et al./2019 | 88 | Not reported | 5 months after index surgery | Anemia, abdominal pain | Small intestine partial resection |
| [12] | Huynh et al./2019 | Not reported | Not reported | 3 years after index surgery | Right lower back pain | Duodenal resection |
| [3] | Singh et al./2019 | 77 | I A | 3.5 years after index surgery | Melena, anemia | Gastroduodenal artery embolization + radiation therapy |
| [13] | Emiloju et al./2020 | 60 | Not reported | 10 months after index surgery | Abdominal pain, melena, generalized weakness | Pancreaticoduodenectomy |
| [4] | Jang et al./2022 | 55 | I B | 6 months after index surgery | Recurrent epigastric pain | Small intestine partial resection |
| Current case | Nikolovski et al./2024 | 88 | II | 14 months after index surgery | Entero-cutaneous fistula | En-block resection of small intestine with abdominal wall |
| Reference number . | Author/year . | Patient age (years) . | Tumor stage . | Timing of metastasis presentation . | Clinical presentation . | Treatment . |
|---|---|---|---|---|---|---|
| [5] | Bosscher et al./1994 | 55 | IV B | Diagnosed during index surgery | – | Small intestine partial resection |
| [6] | Kirk et al./1999 | 64 | Not reported | 10 months after index surgery | Intermittent abdominal cramps and melena | Small intestine partial resection |
| [8] | Thijs et al./2007 | 85 | Not reported | 2 years after index surgery | Anemia | Small intestine partial resection |
| [10] | Tsai et al./2007 | 67 | Early-stage | 8 years after index surgery | Epigastric pain, melena | Segmental duodenectomy with Roux-en-Y duodenojejunostomy |
| [11] | Gallotta et al./2015 | 58 | I B | 13 months after index surgery | Not reported | Laparoscopic small intestine resection |
| [9] | Hubers and Soni/2017 | 75 | I B | 3 years after index surgery | Blood per rectum (synchronous sigmoid colon metastasis) | Small intestine partial resection + low anterior rectal resection |
| [7] | Leitão et al./2017 | 72 | Not reported | 5 years after index surgery | Melena | Palliative chemotherapy |
| [2] | Makki et al./2019 | 88 | Not reported | 5 months after index surgery | Anemia, abdominal pain | Small intestine partial resection |
| [12] | Huynh et al./2019 | Not reported | Not reported | 3 years after index surgery | Right lower back pain | Duodenal resection |
| [3] | Singh et al./2019 | 77 | I A | 3.5 years after index surgery | Melena, anemia | Gastroduodenal artery embolization + radiation therapy |
| [13] | Emiloju et al./2020 | 60 | Not reported | 10 months after index surgery | Abdominal pain, melena, generalized weakness | Pancreaticoduodenectomy |
| [4] | Jang et al./2022 | 55 | I B | 6 months after index surgery | Recurrent epigastric pain | Small intestine partial resection |
| Current case | Nikolovski et al./2024 | 88 | II | 14 months after index surgery | Entero-cutaneous fistula | En-block resection of small intestine with abdominal wall |
| Reference number . | Author/year . | Patient age (years) . | Tumor stage . | Timing of metastasis presentation . | Clinical presentation . | Treatment . |
|---|---|---|---|---|---|---|
| [5] | Bosscher et al./1994 | 55 | IV B | Diagnosed during index surgery | – | Small intestine partial resection |
| [6] | Kirk et al./1999 | 64 | Not reported | 10 months after index surgery | Intermittent abdominal cramps and melena | Small intestine partial resection |
| [8] | Thijs et al./2007 | 85 | Not reported | 2 years after index surgery | Anemia | Small intestine partial resection |
| [10] | Tsai et al./2007 | 67 | Early-stage | 8 years after index surgery | Epigastric pain, melena | Segmental duodenectomy with Roux-en-Y duodenojejunostomy |
| [11] | Gallotta et al./2015 | 58 | I B | 13 months after index surgery | Not reported | Laparoscopic small intestine resection |
| [9] | Hubers and Soni/2017 | 75 | I B | 3 years after index surgery | Blood per rectum (synchronous sigmoid colon metastasis) | Small intestine partial resection + low anterior rectal resection |
| [7] | Leitão et al./2017 | 72 | Not reported | 5 years after index surgery | Melena | Palliative chemotherapy |
| [2] | Makki et al./2019 | 88 | Not reported | 5 months after index surgery | Anemia, abdominal pain | Small intestine partial resection |
| [12] | Huynh et al./2019 | Not reported | Not reported | 3 years after index surgery | Right lower back pain | Duodenal resection |
| [3] | Singh et al./2019 | 77 | I A | 3.5 years after index surgery | Melena, anemia | Gastroduodenal artery embolization + radiation therapy |
| [13] | Emiloju et al./2020 | 60 | Not reported | 10 months after index surgery | Abdominal pain, melena, generalized weakness | Pancreaticoduodenectomy |
| [4] | Jang et al./2022 | 55 | I B | 6 months after index surgery | Recurrent epigastric pain | Small intestine partial resection |
| Current case | Nikolovski et al./2024 | 88 | II | 14 months after index surgery | Entero-cutaneous fistula | En-block resection of small intestine with abdominal wall |
EC spreads by direct invasion via the lymphatic and hematogenous route [3, 5]. The mechanism of EC spread to the small intestine is not fully explained. The time frame of metastasis occurrence according to the literature research varies from 0 months to 8 years from the index surgery.
Small intestine metastases from EC may clinically present with abdominal cramps, melena, secondary anemia, and epigastric pain [3, 6, 7]. In this case, the patient presented with an enterocutaneous fistula and surrounding skin inflammation.
The diagnosis of EC metastasis to the small intestine is delayed for a few weeks to months in most cases. The reported diagnostic tools useful in such cases are plain and contrast radiographs, abdominal CT scans, upper endoscopy, magnetic resonance imaging, and [18F] fluorodeoxyglucose-positron emission tomography (PET) scan [1, 6]. When active bleeding from the metastasis is suspected, CT angiography and scintigraphy with 99mTc-marked red blood cells were described to be useful [3]. More distant metastasis locations unreachable for the endoscope, might be visualized by video capsule endoscopy [8]. Since the metastatic disease was confirmed in this case, an additional PET scan might reveal the existence of extra-intestinal metastases and could be an additional diagnostic tool.
The final diagnosis is confirmed with the pathology analysis. Additional immunohistochemistry staining differentiates intestinal metastases from other primary adenocarcinomas, especially colorectal cancer. EC shows positivity to CK 7, PAX8, Estrogen Receptor, and Progesterone Receptor [7, 9].
The reported EC recurrence treatment is heterogeneous and comprehends palliative radiation, surgery, chemotherapy, hormone therapy, or a certain combination of the above [3]. This treatment should be targeted for symptom relief. Having in mind the possibility of intestinal obstruction occurrence, in cases of small intestine EC metastasis, surgery should be considered in the first place. At the same time, the risk of bleeding from the metastasis will be eliminated. In this case, the patient’s quality of life was reduced due to the enterocutaneous fistula persistence, and surgery was considered the treatment of choice. Following surgery, an oncologist recommended first-line adjuvant chemotherapy since the patient did not receive any after the index surgery. Having in mind the singularity of the small intestine metastasis, longer survival might be expected in comparison with patients with multiple metastases.
This report is the first published case of metastatic EC to the small intestine with enterocutaneous fistula treated successfully with en-block resection. Clinicians should be aware of rare metastasis locations from EC, such as the gastrointestinal ones, which can occur in a wide time frame after the initial treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
None declared.



