-
PDF
- Split View
-
Views
-
Cite
Cite
Kaixin Zhang, Wei Dai, Hongfan Yu, Qiuling Shi, Shaohua Xie, Bin Hu, Qiang Li, Xing Wei, Multidisciplinary management and surgical resection of a rare posterior mediastinal haemangioma, Journal of Surgical Case Reports, Volume 2024, Issue 4, April 2024, rjae225, https://doi.org/10.1093/jscr/rjae225
Close - Share Icon Share
Abstract
Mediastinal haemangiomas pose diagnostic and therapeutic challenges owing to their rarity and complex anatomy. A 36-year-old man, with a history of smoking and drinking, presented with a posterior mediastinal mass with back pain. Initial investigations suggested a lymphangioma. However, owing to persistent symptoms and complex pathology, we performed surgical intervention involving open resection of the tumour, which was closely associated with the descending aorta and extended into the right posterior mediastinum. The surgical approach was influenced by the proximity of the tumour to vital structures, necessitating an open procedure. Postoperative complications included chylothorax, managed with a fat-free diet. The final pathological diagnosis was consistent with a benign vascular tumour with a low proliferative rate. Two months post-surgery, computed tomography revealed no complications, and the patient’s pain had decreased. A multidisciplinary approach and surgical intervention played important roles in the diagnosis and treatment of this posterior mediastinal haemangioma.
Introduction
Mediastinal tumours, encompassing diverse pathologies, present significant diagnostic and therapeutic challenges owing to their anatomical location and varied presentation [1].
Diagnosing mediastinal haemangiomas is challenging, often requiring a combination of imaging modalities and histopathological analysis [1]. Radiographically, these tumours present as non-specific masses, making their differentiation from other mediastinal pathologies difficult [2]. Therefore, biopsy and immunohistochemical analysis play crucial roles in establishing a definitive diagnosis [3]. Notably, surgical resection remains the primary treatment for mediastinal haemangiomas [1, 4, 5]. In complex cases, a multidisciplinary approach involving thoracic surgery, radiology, pathology, and other specialties is essential for optimal management [1, 4].
This case report outlines the management of a rare posterior mediastinal haemangioma, underscoring the necessity of a multidisciplinary strategy and surgical intervention in such cases.
Case report
A 36-year-old male smoker and drinker was hospitalized for a posterior mediastinal mass causing back pain for 4 months, with a 2-year history but no infectious or genetic disease. Initial computed tomography (CT) showed a spindle-shaped mass; he was asymptomatic initially and sought no treatment. Later, a biopsy for back pain showed adipose and fibrous tissue with lymphocytes and plasma cells, indicating a tumour. Immunohistochemistry showed lymphangioma markers (D2–40, CD31, CD34), Desmin (+), heterogeneous CD3 and CD20 lymphocytes, CD38+ plasma cells, increased IgG4 cells (~25/HPF), Ki67 < 5%, and elevated IgG4 level (3.480). Oral Prednisone (60 mg twice daily) was ineffective, leading to discontinuation after 1 month. He sought further evaluation 4 months later.
At that time, evaluation with chest CT and magnetic resonance imaging (MRI) identified a large, strip-like soft tissue mass in the left posterior mediastinum. It was adjacent to the thoracic intervertebral foramen, encircled the descending aorta’s posterior wall, and extended into the right mediastinum, affecting the azygos vein. Positron emission tomography-computed tomography (PET-CT) showed a 65 × 32 mm sized mass with increased uptake (SUV max 2.2). MRI showed a mass with mixed signals on T1WI, high signals on T2WI, and localized cystic areas, suggesting a neurogenic tumour (Fig. 1).
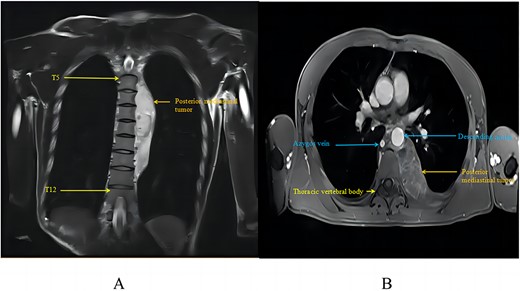
Posterior mediastinal haemangioma as seen on chest MRI: (A) tumour in MRI coronal view; (B) tumour in MRI axial view.
A multidisciplinary treatment (MDT) team recommended surgery for complete tumour removal and accurate diagnosis, involving lymphoma, oncology, thoracic surgery, radiology, pathology, and anaesthesiology specialists. The thoracic surgery experts noted that minimally invasive surgery would be challenging and risky; therefore, open surgery was advised [6, 7]. After a comprehensive discussion encompassing the MDT team’s recommendations, a detailed explanation of the planned surgical approach and an inclusive shared decision-making process involving the patient and his family, the patient provided informed consent and opted to proceed with the surgery [8].
The patient was placed in the right lateral decubitus position, and access to the thoracic cavity was achieved through an incision in the anterior lateral aspect of the fifth intercostal space on the left side. The lesion, located in the left posterior mediastinum, exhibited a space-occupying growth pattern encasing the descending aorta. It was closely associated with the ribs, spine, and descending aorta but did not invade the lung tissue (Fig. 2). Using ultrasound and electrocautery, the tumour was meticulously dissected from the descending aorta, ribs, and spine along its margins. A endoscopic linear cutting stapler was utilized to suture and transect the tumour at its base in the right posterior mediastinum (Fig. 3). After ensuring thorough haemostasis, a chest drain was placed, the lungs were re-expanded, and the incision was closed. The surgery took 195 min with an estimated blood loss of 300 ml. The patient then moved to the thoracic surgery ward for recovery. Intraoperative frozen section analysis of the specimen diagnosed a vascular tumour, as indicated by negative SOX-10 and Ckpan (AE1/AE3) immunohistochemical staining.
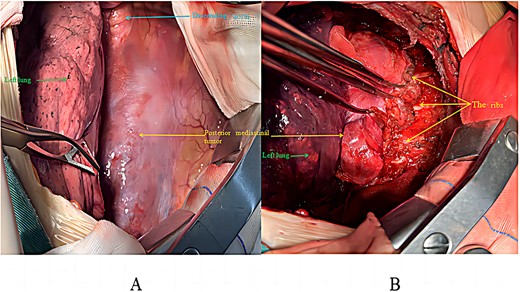
Intraoperative surgical procedure: (A) before tumour excision; (B) during tumour mobilization.
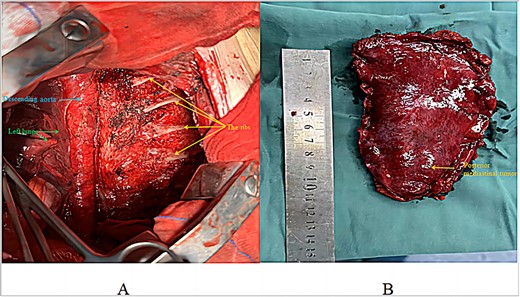
Post-resection surgical view: (A) view after tumour resection; (B) excised tumour specimen.
The patient began a liquid diet 6 h post-surgery, received IV cefazolin sodium for infection prevention until Day 3, and received daily low-molecular-weight heparin injections for thrombosis prevention. On post-surgery Day 1, stable vitals allowed stopping ECG monitoring and catheter removal, enabling ambulation. A chest radiograph revealed good lung re-expansion. Blood tests indicated haemoglobin level at 146 g/L and WBC count at 7.96 × 109/L.
Postoperative recovery involved 420 ml of milky pleural drainage in the first day, suggestive of chylothorax. The patient followed a fat-free diet, leading to the resolution of chylous drainage by Day 12, at which point he returned to a normal diet. A chest CT on Day 14 demonstrated successful lung re-expansion without pleural effusion, permitting the removal of the chest tube. He was discharged the day after. Pathological analysis revealed vascular-like structures with red blood cells, minimal lymphocytes, consistent with a vascular tumour, diagnosed as an angioma (Fig. 4B) after immunohistochemistry tests for SOX-10 and AE1/AE3 were negative.
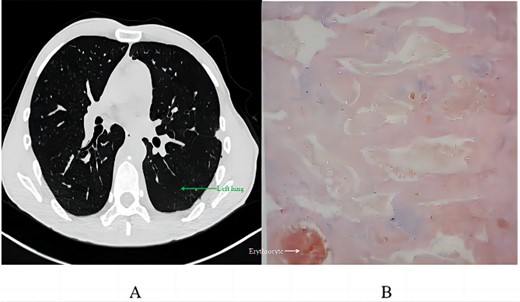
Postoperative analysis: (A) chest CT scan 2 months post-discharge; (B) pathological section.
Two months after discharge, a follow-up CT scan revealed no significant pneumothorax, pleural effusion, or pneumonia, with no abnormal soft tissue in the surgical area. The patient noted a significant decrease in pain since discharge.
Discussion
This case involved a complex diagnosis with imaging and biopsy and was initially misidentified as lymphangioma. The correct angioma diagnosis was made after surgery, underlining the role of surgical intervention in diagnosing and treating complex mediastinal tumours. Successful resection, despite tumour extension into the right posterior mediastinum, demonstrates the feasibility and safety of careful surgical planning and execution in such complex cases.
Chylothorax, a notable complication, developed post-surgically, likely because of lymphatic vessel disruption during tumour resection. This was managed with a fat-free diet until resolution, consistent with literature on chylothorax management [9]. The pathological findings of a vascular tumour, specifically an angioma, are consistent with the origin and behaviour of the tumour. The low Ki67 index indicated a low proliferative rate, typical of benign vascular lesions [10]. The absence of SOX-10 and pan-cytokeratin (AE1/AE3) further supported the non-neurogenic, non-epithelial nature of the tumour. The 2-month follow-up, showing no significant complications or recurrence, is promising. However, given the rarity of these tumours and the potential for recurrence, long-term surveillance is recommended [11, 12].
Conclusion
This case highlights the diagnostic and therapeutic challenges posed by rare posterior mediastinal haemangiomas, the importance of a multidisciplinary approach in managing complex mediastinal masses, and the role of surgical intervention in both diagnosis and treatment.
Acknowledgements
We sincerely thank the patient for their cooperation during treatment and for their consent to publish this case. Additionally, we acknowledge Dong Yuan’s invaluable support in revising the manuscript.
Conflict of interest statement
None declared.
Funding
This work was supported by the Natural Science Foundation of Sichuan Province (No. 2023NSFSC1047). The funder had no involvement in the data collection, analysis, interpretation, manuscript composition, or the decision to submit the paper for publication.
Data availability
The data sets used are available from the corresponding author upon request.
References
Author notes
Qiang Li and Xing Wei contributed equally to this work as corresponding authors.



