-
PDF
- Split View
-
Views
-
Cite
Cite
Yoko Nakayama, Minekazu Yamaguchi, Keisuke Inoue, Masaki Sasaki, Kaho Tamaki, Masaaki Hidaka, Well-leg compartment syndrome after laparoscopic low anterior resection in the lithotomy position: a case report and literature review, Journal of Surgical Case Reports, Volume 2024, Issue 4, April 2024, rjae206, https://doi.org/10.1093/jscr/rjae206
Close - Share Icon Share
Abstract
Well-leg compartment syndrome (WLCS) develops in healthy lower limbs because of surgical factors such as operative position, lower limb compression, and long operative time during abdominopelvic surgery. WLCS can lead to irreversible muscle and nerve damage if a prompt diagnosis and appropriate treatment are not provided. We report the case of a 57-year-old male who developed rectal cancer immediately after laparoscopic low anterior resection and was successfully treated with fasciotomy without sequelae. Patients who undergo surgery in the lithotomy position for a prolonged period are at risk of WLCS. Therefore, when determining the differential diagnosis of postoperative lower leg pain, it is necessary to consider WLCS because it is a complication caused by the intraoperative position.
Introduction
Well-leg compartment syndrome (WLCS) is an increase in the intracompartmental pressure on the extremities, resulting in decreased perfusion pressure, tissue ischemia, and necrosis [1, 2]. While WLCS is rare [3, 4], its implications can be severe, leading to permanent paresthesia, renal dysfunction, and even death [4, 5] if timely diagnosis and appropriate interventions are not provided [1]. WLCS risks are broadly categorized into patient related (sex, age, obesity, and peripheral vascular disease) and intraoperative (abdominopelvic surgery, intraoperative positioning, prolonged operative time, use of antiembolism stockings, and fluid and blood pressure management) [1, 3, 6]. Urologists, obstetricians, gynecologists, and surgeons performing abdominopelvic surgeries involving lithotomy positioning should understand and prevent WLCS. Here, we present a case of WLCS manifesting immediately after laparoscopic low anterior rectal cancer resection [7].
Case report
A 57-year-old man [height, 164.9 cm; weight, 71.4 kg; body mass index (BMI), 26.3] was diagnosed with adenocarcinoma in the lower rectum. He was using medications for hypertension and hyperuricemia, which were well-controlled. Laparoscopic low anterior resection and D3 dissection were performed under general and epidural anesthesia. To prevent thrombosis, surgical stockings, up to the knee, and an intermittent pneumatic compression (IPC) device were placed only on the soles of the feet. Surgery was performed in the lithotomy position using a Levitator® (Mizuno, Tokyo, Japan) with gel pads, with the head in a low position and rotated to the right. The operative time was 507 min, blood loss was 200 mL, and the urine was slightly brown (total 320 mL). His blood pressure remained stable during surgery.
Immediately after surgery, he experienced numbness in the left lower leg that progressed to severe pain 3 h later. Plantar dorsiflexion of the ankle joint was possible, and the left lower leg was tense; however, the dorsalis pedis artery was palpable. Abnormalities in the lower leg diameter and color tone were not observed. Abnormally high muscle lactate dehydrogenase (1084 IU/L) and serum creatine kinase (CK) (23 571 IU/L) levels were observed 8 h postoperatively. His dark brown urine suggested myoglobinuria (not measured). Contrast-enhanced computed tomography (15 h after surgery) revealed no obvious lower extremity venous thrombosis; however, edema in the soleus and gastrocnemius muscles of the left lower leg suggested a diagnosis of left lower leg compartment syndrome (CS) (Fig. 1). Calf muscle pressure monitoring was not possible in our hospital; therefore, he was clinically diagnosed with WLCS.
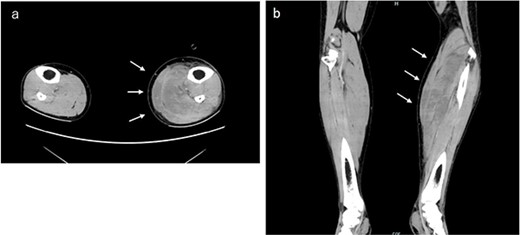
Contrast-enhanced computed tomography examination of both lower limbs. Obvious venous thrombosis was not observed in the lower extremity vessels. Significant edema was observed in the soleus and gastrocnemius muscles of the left lower leg. (a) Horizontal and (b) coronal sections.
After consultation with an orthopedic surgeon at our hospital, decompression was deemed necessary. Eighteen hours after surgery, he was transported to a higher-level medical institution, and a decompression incision was performed. WLCS progression was uneventful; at 1 month after surgery, there were no sequelae related to lower limb motion.
Discussion
Acute CS of the lower limb, associated with surgical positions including lithotomy-Trendelenburg or Lloyd-Davies, is a severe complication [1–3] commonly known as WLCS because it can occur in healthy lower limbs [8]. The incidence of WLCS post-surgery in the lithotomy position ranges from 0.03% to 0.2% [4, 8]. Stoodley and Thomson [9] first reported CS associated with colorectal surgery. Additionally, Peters [5] reported nine cases associated with abdominopelvic surgery. The incidence of such cases has gradually increased with the expansion of laparoscopic and robot-assisted rectal surgery [10].
Lower limb CS is attributed to the reperfusion of ischemic or hypoxic tissues [1, 2, 8, 10]. The two factors that cause ischemia and can trigger WLCS are external compression and hypoperfusion [11]. Surgical stockings [1] and IPC [1, 3] exerted external pressure on the lower limbs, decreasing blood flow to the lower limbs. Reduced peripheral blood flow leads to muscle and nerve ischemia [11]. Hypoperfusion has various causes, including leg elevation during the surgical positioning [12, 13], intraoperative hypotension [5], low circulating blood volume, hypothermia, vasoconstrictors, spinal anesthesia, a history of peripheral vascular disease, and traction of large vessels during surgery [11].
Patient-related risk factors associated with WLCS include male sex [6], younger age (<60 years) [14], and obesity (BMI ≥ 25 kg/m2) [6, 13]. According to Sajid et al. [14], the average age of WLCS onset is as young as 35.5 years. Young individuals may be more susceptible to WLCS because of their muscle bulk and tight, less-compliant compartments [3].
Symptoms typically occur bilaterally or unilaterally [1]. The lower extremities comprise four compartments (anterior, lateral, superficial posterior, and deep posterior). The pentalogy of WLCS comprises pallor, disproportionate pain, pulselessness, paresthesia, and paralysis. The most characteristic symptom is pain [2]. Although intracompartmental pressure is often measured, clinical diagnosis is often sufficient; therefore, making invasive testing not routinely recommended [1]. Additionally, laboratory investigations, particularly CK levels, can promptly measure tissue damage caused by WLCS. Elevated CK levels (≥4000 U/L) can indicate severe muscle damage and aid in WLCS diagnosis [1]. Measurement of CK levels on the first postoperative day may be important for WLCS screening. MRI, especially T2-weighted imaging, is effective for diagnosing muscle edema [15].
The primary treatment for WLCS is the prompt reduction of the increased compartment pressure to alleviate tissue damage that can lead to ischemia. Therefore, comprehensive treatment requires releasing all four compartments of the lower limb [1, 3, 8]. A delayed in fasciotomy beyond 12 h [1] raises the risk and incidence of irreversible sequelae; however, in most cases, full recovery can be achieved if fasciotomy is performed within 6 h of diagnosis [1].
To reduce WLCS risk, shortening the operative time through improved surgical techniques is crucial. Some studies have suggested that repositioning the lower limbs horizontally every 2–3 h should be performed during prolonged surgery [13]. Based on our experience at our hospital, when securing the lower limbs during surgery, we checked whether unnecessary loads were applied to both the lower limbs, checked the size of the elastic stockings, and appropriately used the lifting devices. Furthermore, we checked the lower limbs for compression every 3 h and performed decompression, preventing further WLCS cases.
WLCS is a complication associated with serious sequelae of abdominopelvic surgery performed in the lithotomy position. Therefore, the incidence and sequelae of WLCS should be reduced by performing measures such as appropriate surgical positioning, preoperative preparation, prompt diagnosis, and appropriate treatment.
Author contributions
Yoko Nakayama (drafted the manuscript, performed surgery), Keisuke Inoue and Minekazu Yamaguchi (performed surgery), Masaaki Hidaka (supervised and approved the final version of this manuscript). All authors have read and approved the final version of this manuscript.
Conflict of interest statement
None declared.
Funding
None declared.
Data availability
All data generated or analyzed during this study are included in this published article. Additionally, a summary of this paper was presented at the 77th Annual Meeting of the Japan Society of Coloproctology.



