-
PDF
- Split View
-
Views
-
Cite
Cite
Sarra Chadli, Mohammed Y Oudrhiri, Mouna Maamar, Mahjouba Boutarbouch, Hajar Khibri, Soukaina Haidouri, Ola Messaoud, Omar El-Aoufir, Adyl Melhaoui, Wafaa Ammouri, Abdessamad Ouahabi, Hicham Harmouche, Mohammed Adnaoui, Zoubida Tazi Mezalek, Sphenoid plasmacytoma as initial presentation of multiple myeloma—case report, Journal of Surgical Case Reports, Volume 2024, Issue 4, April 2024, rjae199, https://doi.org/10.1093/jscr/rjae199
Close - Share Icon Share
Abstract
Plasmacytoma is a rare plasma cell neoplasm. Whether solitary or associated with multiple myeloma (MM), it rarely involves the skull base, particularly the sphenoid bone. We present a unique case of sphenoid bone plasmacytoma secondary to MM, highlighting diagnostic and therapeutic challenges. A 56-year-old female presented with headaches, vomiting, epistaxis, and cranial nerve deficits. Cerebral imaging revealed a 65-mm tumor infiltrating the sphenoid bone and adjacent structures. Subtotal resection was performed using an endoscopic nasal approach. Histopathology revealed plasmacytoma, and diagnostic workup confirmed MM. By the end of biological exploration, relapse of the sphenoid plasmacytoma was observed, and the patient was successfully treated with radiotherapy, immunochemotherapy, and autologous stem cell transplantation. After 18-month follow-up, sustained complete remission was confirmed. Although rare, the diagnosis of plasmacytoma should be considered in cases of skull base tumors. This localization is highly predictive of MM, warranting comprehensive investigations to initiate prompt and adequate management.
Introduction
Plasmacytoma is a rare neoplasm characterized by the localized proliferation of a single clone of plasma cells. It may be solitary or associated with multiple myeloma (MM), eventually being its initial presentation. Whether originating from bone or soft tissue, intracranial involvement of plasmacytoma is an unusual phenomenon, particularly at the skull base [1].
Clinical and radiological differentiation from other regional tumors is often challenging, potentially leading to misdiagnosis. Given the notable differences in therapeutic strategies and outcomes, timely recognition of plasmacytoma is of utmost importance.
Here, we present an illustrative case of a patient with multiple cranial nerve deficits and signs of increased intracranial pressure revealing a skull base sphenoid bone plasmacytoma (BP), secondary to MM. This case highlights the complexity involved in diagnosing and managing such patients.
Case report
A 56-year-old female patient, presented with a 2-week history of moderate headaches, intermittent vomiting, epistaxis, and blurred vision. Physical examination revealed decreased visual acuity (5/10 left eye, 7/10 right eye) and bilateral papilledema, with left peripheral facial palsy and trigeminal hypoesthesia.
Cerebral computed tomography (CT) revealed a 65-mm hyperdense tumor invading the sphenoid bone and sinus, and eroding the adjacent skull base and clivus. On magnetic resonance imaging (MRI), the tumor appeared isointense on T1 and hypointense on T2-weighted images, showing mild heterogeneous enhancement after gadolinium injection. Local compression and extension to the left cavernous sinus, internal carotid artery, and optic chiasm were observed (Fig. 1).
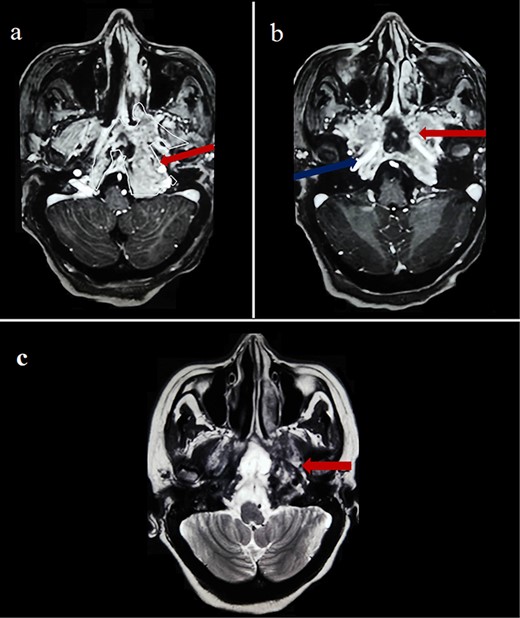
Cerebral MRI (axial views) showing a 65-mm lesion centered on the sphenoid bone (red arrow) with extension to the internal carotid artery (blue arrow), demonstrating iso-intensity with mild heterogeneous enhancement on T1-weighted (a, b) and hypo-intensity on T2-weighted (c).
The patient underwent an endoscopic endonasal approach for debulking and cranial nerve decompression, which revealed a relatively soft, multilayered, reddish tumor, with moderate bleeding. Per-operative frozen section analysis was inconclusive. Resection was conducted to near complete with satisfactory decompression of nervous structures, as confirmed by postoperative cerebral CT, leading to significant improvement in neurological symptoms.
Histopathological examination revealed monoclonal proliferation of dystrophic plasma cells, thereby identifying plasmacytoma (Fig. 2). Serum protein immunoelectrophoresis showed a narrow peak in gammaglobulins (15 g/l) with a monoclonal IgG kappa band. Serum free light chain (sFLC) kappa was elevated at 299 mg/L, with lambda at 78 mg/l, and a K/L ratio of 3.8. Bone marrow aspiration did not reveal abnormal plasma cells or high-risk cytogenetic abnormalities. Bence–Jones proteinuria was negative. Diffuse osteolytic lesions were visualized in the spine. There was no associated anemia, hypercalcemia, or renal failure. Albumin, beta-2-microglobulin, and lactate dehydrogenase levels were normal. Subsequently, the patient was diagnosed with stage I IgG kappa MM.
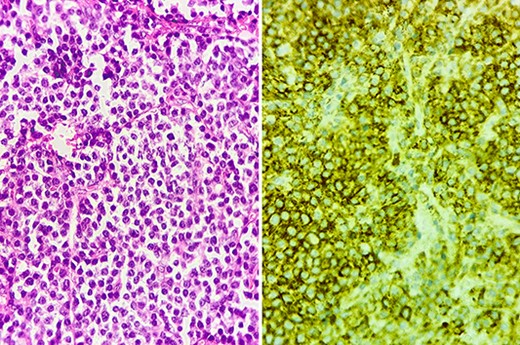
Histology section showing diffuse dystrophic plasma cells predominantly mature (left), with positive CD138 staining on immunohistochemistry (right).
By the end of biological exploration, the patient reported a recurrence of headaches and blurred vision, 2 months after surgical decompression. Cerebral MRI revealed a relapse of the sphenoid plasmacytoma, measuring 70 mm (Fig. 3). Given the pathological diagnostic result, surgical re-intervention was not decided. Instead, the patient was treated with external beam radiotherapy (RTX) focused on the sphenoid bone (30 Gy), followed by immunochemotherapy with zoledronic acid. After 6 months, complete response was noted with the resolution of symptoms, disappearance of the monoclonal band, normalization of sFLC, and persistence of lytic sequelae on cerebral MRI (Fig. 4). Autologous stem cell transplantation (ASCT) was successfully performed, and the patient was put under treatment maintenance. After 18 months of follow-up, clinical, biological, and radiological evaluation confirmed sustained complete remission.
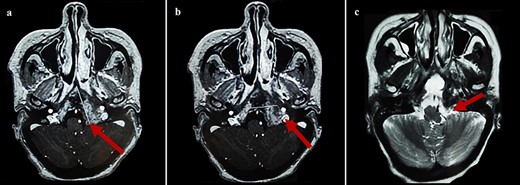
Cerebral MRI (axial views) showing a relapse of the sphenoid BP measuring 70 mm (a, b: T1 weighted post-gadolinium; c: T2 weighted).
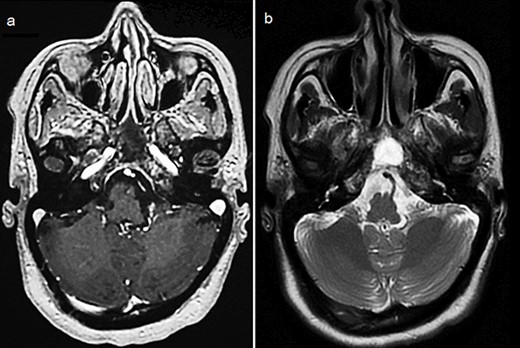
Control cerebral MRI (axial views) showing the disappearance of the sphenoid BP after treatment with RTX and immunochemotherapy. (a: T1-weighted; b: T2-weighted).
Discussion
We report the case of a 56-year-old female patient who presented with headaches, intermittent vomiting, epistaxis, and multiple cranial nerve deficits attributed to a sphenoid bone mass. Surgical resection was performed, and histological examination identified a plasmacytoma secondary to IgG kappa MM. Following relapse, the initiation of RTX, immunochemotherapy, and ASCT enabled sustained clinical remission.
Plasma neoplasms are a group of hematological disorders defined by the clonal proliferation of plasma cells [2]. Plasmacytoma, the localized form (<4%), originates from osseous/intramedullary sites, called “BP,” or from non-osseous/extramedullary sites, called “extramedullary plasmacytoma” (EMP) [3]. BP is more frequent (70%), mainly involving the spine (30%–40%) and long bones (20%–40%) [4, 5]. However, intracranial involvement is uncommon, especially at the skull base. Only 14 cases of sphenoid plasmacytoma were reported, two associated with MM (Table 1) [1].
| Case n° . | Authors (year) . | Sex . | Age (years) . | Symptoms . | Primary site . | PCN . | Treatment . | Evolution (months) . |
|---|---|---|---|---|---|---|---|---|
| 1 | Losa et al. [18] (1992) | F | 50 | Diplopia | Sphenoid sinus | SP | Resection + RTX | LC |
| 2 | Marais et al. [19] (1992) | M | 64 | Headaches, diplopia | Sphenoid sinus | SP | RTX | LC (18) |
| 3 | Bindal et al. [20] (1995) | F | 51 | Diplopia | Sphenoid +clivus | SP | Resection + RTX | LC (96) |
| 4 | Miller et al. [21] (1998) | M | 47 | Headaches | Sphenoid sinus | SP | RTX | LC (62) |
| 5 | Mandagere et al. [22] (1998) | F | 53 | Headaches, blurry vision | Sphenoid | SP | RTX | LC (84) |
| 6 | Wein et al. [23] (2002) | M | 48 | Headaches, epistaxis | Sphenoid sinus +clivus | MM | RTX + CTX | ER |
| 7 | Akhaddar et al. [24] (2008) | M | 83 | Headaches, diplopia | Sphenoid | MM | RTX + CTX | ND |
| 8 | Lakhdar et al. [25] (2008) | F | 62 | Headaches, diplopia, anosmia | Sphenoid | SP | RTX | LC (12) |
| 9 | Park et al. [26] (2009) | M | 32 | Diplopia | Sphenoid | SP | Resection + RTX | LC (8) |
| 10 | Siyag et al. [27] (2018) | M | 41 | Headaches, diplopia, strabismus | Sphenoid +clivus | SP | RTX | LC (3) |
| 11 | Loong et al. [28] (2019) | F | 43 | Headaches, diplopia | Sphenoid | SP | RTX | ND |
| 12 | Sharifi et al. [29] (2022) | F | 62 | Headaches, ptosis, visual loss | Sphenoid | SP | Resection + RTX | LC (6) |
| 13 | Ampil et al. [30] (2022) | M | 63 | Headaches, diplopia | Sphenoid sinus | SP | Resection + RTX + CTX | LC (3) |
| 14 | Mansouri et al. [31] (2023) | F | 51 | Headaches, diplopia, visual loss, epistaxis | Sphenoid +clivus | SP | RTX | LC (12) |
| Our case | F | 56 | Headaches, vomiting, blurry vision, epistaxis | Sphenoid + clivus | MM | Resection + RTX + CTX + ASCT | CR (18) | |
| Case n° . | Authors (year) . | Sex . | Age (years) . | Symptoms . | Primary site . | PCN . | Treatment . | Evolution (months) . |
|---|---|---|---|---|---|---|---|---|
| 1 | Losa et al. [18] (1992) | F | 50 | Diplopia | Sphenoid sinus | SP | Resection + RTX | LC |
| 2 | Marais et al. [19] (1992) | M | 64 | Headaches, diplopia | Sphenoid sinus | SP | RTX | LC (18) |
| 3 | Bindal et al. [20] (1995) | F | 51 | Diplopia | Sphenoid +clivus | SP | Resection + RTX | LC (96) |
| 4 | Miller et al. [21] (1998) | M | 47 | Headaches | Sphenoid sinus | SP | RTX | LC (62) |
| 5 | Mandagere et al. [22] (1998) | F | 53 | Headaches, blurry vision | Sphenoid | SP | RTX | LC (84) |
| 6 | Wein et al. [23] (2002) | M | 48 | Headaches, epistaxis | Sphenoid sinus +clivus | MM | RTX + CTX | ER |
| 7 | Akhaddar et al. [24] (2008) | M | 83 | Headaches, diplopia | Sphenoid | MM | RTX + CTX | ND |
| 8 | Lakhdar et al. [25] (2008) | F | 62 | Headaches, diplopia, anosmia | Sphenoid | SP | RTX | LC (12) |
| 9 | Park et al. [26] (2009) | M | 32 | Diplopia | Sphenoid | SP | Resection + RTX | LC (8) |
| 10 | Siyag et al. [27] (2018) | M | 41 | Headaches, diplopia, strabismus | Sphenoid +clivus | SP | RTX | LC (3) |
| 11 | Loong et al. [28] (2019) | F | 43 | Headaches, diplopia | Sphenoid | SP | RTX | ND |
| 12 | Sharifi et al. [29] (2022) | F | 62 | Headaches, ptosis, visual loss | Sphenoid | SP | Resection + RTX | LC (6) |
| 13 | Ampil et al. [30] (2022) | M | 63 | Headaches, diplopia | Sphenoid sinus | SP | Resection + RTX + CTX | LC (3) |
| 14 | Mansouri et al. [31] (2023) | F | 51 | Headaches, diplopia, visual loss, epistaxis | Sphenoid +clivus | SP | RTX | LC (12) |
| Our case | F | 56 | Headaches, vomiting, blurry vision, epistaxis | Sphenoid + clivus | MM | Resection + RTX + CTX + ASCT | CR (18) | |
PCN: plasma cell neoplasm; M: male; F: female; MM: multiple myeloma; SP: solitary plasmacytoma; RTX: radiotherapy; CTX: chemotherapy; CR: complete remission; LC: local control; ER: early response; ND: non-documented.
| Case n° . | Authors (year) . | Sex . | Age (years) . | Symptoms . | Primary site . | PCN . | Treatment . | Evolution (months) . |
|---|---|---|---|---|---|---|---|---|
| 1 | Losa et al. [18] (1992) | F | 50 | Diplopia | Sphenoid sinus | SP | Resection + RTX | LC |
| 2 | Marais et al. [19] (1992) | M | 64 | Headaches, diplopia | Sphenoid sinus | SP | RTX | LC (18) |
| 3 | Bindal et al. [20] (1995) | F | 51 | Diplopia | Sphenoid +clivus | SP | Resection + RTX | LC (96) |
| 4 | Miller et al. [21] (1998) | M | 47 | Headaches | Sphenoid sinus | SP | RTX | LC (62) |
| 5 | Mandagere et al. [22] (1998) | F | 53 | Headaches, blurry vision | Sphenoid | SP | RTX | LC (84) |
| 6 | Wein et al. [23] (2002) | M | 48 | Headaches, epistaxis | Sphenoid sinus +clivus | MM | RTX + CTX | ER |
| 7 | Akhaddar et al. [24] (2008) | M | 83 | Headaches, diplopia | Sphenoid | MM | RTX + CTX | ND |
| 8 | Lakhdar et al. [25] (2008) | F | 62 | Headaches, diplopia, anosmia | Sphenoid | SP | RTX | LC (12) |
| 9 | Park et al. [26] (2009) | M | 32 | Diplopia | Sphenoid | SP | Resection + RTX | LC (8) |
| 10 | Siyag et al. [27] (2018) | M | 41 | Headaches, diplopia, strabismus | Sphenoid +clivus | SP | RTX | LC (3) |
| 11 | Loong et al. [28] (2019) | F | 43 | Headaches, diplopia | Sphenoid | SP | RTX | ND |
| 12 | Sharifi et al. [29] (2022) | F | 62 | Headaches, ptosis, visual loss | Sphenoid | SP | Resection + RTX | LC (6) |
| 13 | Ampil et al. [30] (2022) | M | 63 | Headaches, diplopia | Sphenoid sinus | SP | Resection + RTX + CTX | LC (3) |
| 14 | Mansouri et al. [31] (2023) | F | 51 | Headaches, diplopia, visual loss, epistaxis | Sphenoid +clivus | SP | RTX | LC (12) |
| Our case | F | 56 | Headaches, vomiting, blurry vision, epistaxis | Sphenoid + clivus | MM | Resection + RTX + CTX + ASCT | CR (18) | |
| Case n° . | Authors (year) . | Sex . | Age (years) . | Symptoms . | Primary site . | PCN . | Treatment . | Evolution (months) . |
|---|---|---|---|---|---|---|---|---|
| 1 | Losa et al. [18] (1992) | F | 50 | Diplopia | Sphenoid sinus | SP | Resection + RTX | LC |
| 2 | Marais et al. [19] (1992) | M | 64 | Headaches, diplopia | Sphenoid sinus | SP | RTX | LC (18) |
| 3 | Bindal et al. [20] (1995) | F | 51 | Diplopia | Sphenoid +clivus | SP | Resection + RTX | LC (96) |
| 4 | Miller et al. [21] (1998) | M | 47 | Headaches | Sphenoid sinus | SP | RTX | LC (62) |
| 5 | Mandagere et al. [22] (1998) | F | 53 | Headaches, blurry vision | Sphenoid | SP | RTX | LC (84) |
| 6 | Wein et al. [23] (2002) | M | 48 | Headaches, epistaxis | Sphenoid sinus +clivus | MM | RTX + CTX | ER |
| 7 | Akhaddar et al. [24] (2008) | M | 83 | Headaches, diplopia | Sphenoid | MM | RTX + CTX | ND |
| 8 | Lakhdar et al. [25] (2008) | F | 62 | Headaches, diplopia, anosmia | Sphenoid | SP | RTX | LC (12) |
| 9 | Park et al. [26] (2009) | M | 32 | Diplopia | Sphenoid | SP | Resection + RTX | LC (8) |
| 10 | Siyag et al. [27] (2018) | M | 41 | Headaches, diplopia, strabismus | Sphenoid +clivus | SP | RTX | LC (3) |
| 11 | Loong et al. [28] (2019) | F | 43 | Headaches, diplopia | Sphenoid | SP | RTX | ND |
| 12 | Sharifi et al. [29] (2022) | F | 62 | Headaches, ptosis, visual loss | Sphenoid | SP | Resection + RTX | LC (6) |
| 13 | Ampil et al. [30] (2022) | M | 63 | Headaches, diplopia | Sphenoid sinus | SP | Resection + RTX + CTX | LC (3) |
| 14 | Mansouri et al. [31] (2023) | F | 51 | Headaches, diplopia, visual loss, epistaxis | Sphenoid +clivus | SP | RTX | LC (12) |
| Our case | F | 56 | Headaches, vomiting, blurry vision, epistaxis | Sphenoid + clivus | MM | Resection + RTX + CTX + ASCT | CR (18) | |
PCN: plasma cell neoplasm; M: male; F: female; MM: multiple myeloma; SP: solitary plasmacytoma; RTX: radiotherapy; CTX: chemotherapy; CR: complete remission; LC: local control; ER: early response; ND: non-documented.
BP typically manifests with variable mass effect, pain, and infiltrative behavior [6]. When located at the skull, it may grow asymptomatic, only manifesting when reaching large sizes and infiltrating neural elements, as was observed in our case [7]. The patient presented multiple cranial nerve deficits mainly attributed to a direct compressive effect. These findings are usually present in tumors involving the sphenoid bone, petrous bone, and cavernous sinus [5].
Radiologically, BP appears as a well-circumscribed mass on CT scan, often associated with lytic lesions and infiltration of adjacent structures. On MRI, discrete heterogeneity is generally observed, with iso to hyperintensity on T1 and iso to hypointensity on T2-weighted images, and mild to vivid homogeneous contrast enhancement [8, 9]. Yet, these radiological findings are not specific to plasmacytoma and may be difficult to differentiate from other regional tumors (chordoma, chondroma, carcinomas, etc.) [10].
The diagnosis of plasmacytoma is established on immunohistological examination showing diffuse infiltration by monoclonal dystrophic plasma cells. Serum and urine immunoelectrophoresis must be performed to detect monoclonal protein [11]. To differentiate solitary plasmacytoma (SP; Table 2) from MM, bone marrow involvement and organ damage evidence that can be attributed to MM, including additional sites of bone lesions, must be investigated (Table 3) [12, 13]. If MM is confirmed, risk stratification is needed to further guide management (Table 4).
| All four criteria must be met . |
|---|
| – Biopsy-proven solitary lesion of bone or soft tissue with evidence of clonal plasma cells |
| – Normal bone marrow with no evidence of clonal plasma cells |
| – Normal skeletal survey and MRI (or CT) of spine and pelvis (except for the primary solitary lesion) |
| – Absence of end-organ damage such as hypercalcemia, renal insufficiency, anemia, or bone lesions (CRAB) that can be attributed to a lympho-plasma cell proliferative disorder |
| All four criteria must be met . |
|---|
| – Biopsy-proven solitary lesion of bone or soft tissue with evidence of clonal plasma cells |
| – Normal bone marrow with no evidence of clonal plasma cells |
| – Normal skeletal survey and MRI (or CT) of spine and pelvis (except for the primary solitary lesion) |
| – Absence of end-organ damage such as hypercalcemia, renal insufficiency, anemia, or bone lesions (CRAB) that can be attributed to a lympho-plasma cell proliferative disorder |
| All four criteria must be met . |
|---|
| – Biopsy-proven solitary lesion of bone or soft tissue with evidence of clonal plasma cells |
| – Normal bone marrow with no evidence of clonal plasma cells |
| – Normal skeletal survey and MRI (or CT) of spine and pelvis (except for the primary solitary lesion) |
| – Absence of end-organ damage such as hypercalcemia, renal insufficiency, anemia, or bone lesions (CRAB) that can be attributed to a lympho-plasma cell proliferative disorder |
| All four criteria must be met . |
|---|
| – Biopsy-proven solitary lesion of bone or soft tissue with evidence of clonal plasma cells |
| – Normal bone marrow with no evidence of clonal plasma cells |
| – Normal skeletal survey and MRI (or CT) of spine and pelvis (except for the primary solitary lesion) |
| – Absence of end-organ damage such as hypercalcemia, renal insufficiency, anemia, or bone lesions (CRAB) that can be attributed to a lympho-plasma cell proliferative disorder |
| Both criteria must be met: . |
|---|
| – Clonal bone marrow plasma cells ≥10% or biopsy-proven bony or EMP |
– Any one or more of the following myeloma-defining events:
|
| Both criteria must be met: . |
|---|
| – Clonal bone marrow plasma cells ≥10% or biopsy-proven bony or EMP |
– Any one or more of the following myeloma-defining events:
|
Abbreviations: Ca: serum calcium; Hb: hemoglobin; PET-CT: positron emission tomography-CT; FLC: free light chains; sFLC: serum free light chains.
| Both criteria must be met: . |
|---|
| – Clonal bone marrow plasma cells ≥10% or biopsy-proven bony or EMP |
– Any one or more of the following myeloma-defining events:
|
| Both criteria must be met: . |
|---|
| – Clonal bone marrow plasma cells ≥10% or biopsy-proven bony or EMP |
– Any one or more of the following myeloma-defining events:
|
Abbreviations: Ca: serum calcium; Hb: hemoglobin; PET-CT: positron emission tomography-CT; FLC: free light chains; sFLC: serum free light chains.
| Stages . | Criteria . |
|---|---|
| Stage I | All the following:
|
| Stage II | Not fitting Stage I or III |
| Stage III | Both of the following:
|
| Stages . | Criteria . |
|---|---|
| Stage I | All the following:
|
| Stage II | Not fitting Stage I or III |
| Stage III | Both of the following:
|
Abbreviations: t: translocation; del: deletion.
| Stages . | Criteria . |
|---|---|
| Stage I | All the following:
|
| Stage II | Not fitting Stage I or III |
| Stage III | Both of the following:
|
| Stages . | Criteria . |
|---|---|
| Stage I | All the following:
|
| Stage II | Not fitting Stage I or III |
| Stage III | Both of the following:
|
Abbreviations: t: translocation; del: deletion.
Approximately 50% of plasmacytomas progress to MM, with a higher risk for BP (60%–70%) than EMP (10%–20%) [6]. Moreover, its localization at the skull base is highly predictive of MM. However, our case is only the third reported of sphenoid plasmacytoma related to MM. Other risk factors correlated with this progression include age over 40 years, lesion > 5 cm, high monoclonal protein level, and persistence of residual disease after treatment [14, 15].
Because plasmacytomas are highly radiosensitive, RTX represents the mainstay of management, with or without surgery [16, 17]. Lesions at the skull base may be treated by subtotal resection followed by irradiation or by irradiation alone. Immunochemotherapy, with ASCT for eligible patients, is indicated in refractory plasmacytomas and MM [12, 13].
Although the prognosis of SP is favorable, long-term follow-up is required because of the risk of progression. The survival of patients with MM ranges from several months to more than 10 years, with a 3-year median life expectancy for skull base plasmacytomas. However, patient outcomes have drastically improved in recent decades with novel immunochemotherapy and ASCT [1, 12, 13].
Conclusion
Although rare, sphenoid BP should be considered in the differential diagnosis of skull base tumors. This localization is highly predictive of MM, which needs to be thoroughly investigated. Our patient achieved sustained complete remission following RTX, immunochemotherapy, and ASCT.
Conflict of interest statement
None declared.
Funding
None declared.



