-
PDF
- Split View
-
Views
-
Cite
Cite
Domenico Pinelli, Claudio Guerci, Francesco Cammarata, Riccardo Cirelli, Agnese Scatigno, Michele Colledan, Huge mesenchymal hamartoma in a young adult: a case report, Journal of Surgical Case Reports, Volume 2024, Issue 4, April 2024, rjae184, https://doi.org/10.1093/jscr/rjae184
Close - Share Icon Share
Abstract
Mesenchymal hamartoma of the liver (MHL) is rare. Less than 50 adult cases have been described. Due to their potential degeneration or recurrence, a complete surgical resection must be performed. We describe a case of a 26-year-old with a palpable solid lesion, which displaced abdominal organs. Percutaneous needle biopsies suggested the diagnosis of MHL. A right hemi-hepatectomy without segment 1 was performed; the post-operative course was uneventful. The mesenchymal component of the tumour was reactive to desmin and smooth muscle actin. Low proliferation index was confirmed (MIB1). Genetic counselling: the sequencing analysis of DICER1 and CDKN1C gene was negative, DNA methylation analysis on the chromosome 11p15 region was normal. After 42 months, there was no recurrence. In conclusion, clinicians should consider MHL in the differential diagnosis. The dimension and the need of radicality impose major liver resections or liver transplantations, which should be performed in referral centres.
Introduction
Mesenchymal tumours of the liver arise from vascular, fibrous, adipose, or any other mesenchymal tissue components. They are rare but represent a significant proportion of liver neoplasms, among neonates, infants, and young children. Haemangiomas are the most common mesenchymal liver tumours with a reported incidence of 1–6% [1, 2]. Mesenchymal hamartomas represent the second most frequent benign liver tumour in paediatric population, but <50 cases have been described in adults since 1903 when Maresch reported the first mesenchymal hamartoma of the liver (MHL) [3, 4]. About 75% of MHL affect the right lobe of the liver with a typical clinical picture that includes gastrointestinal symptoms, weight loss, anorexia, malnutrition, palpable homogeneous abdominal mass, and respiratory distress [5]. MHL is considered a pre-malignant tumour by some authors due to its potential degeneration or recurrence as an undifferentiated embryonic sarcoma [6] and for this reason, a complete surgical resection even with liver transplantation should always be pursued [7].
Presentation of the case
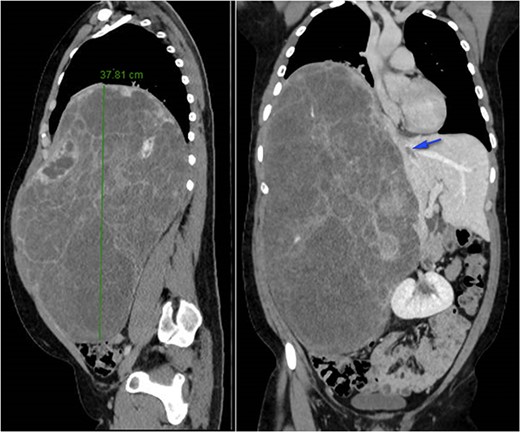
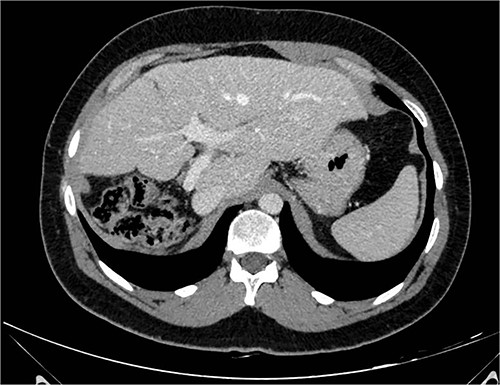
A 26-year-old, previously healthy man was admitted in March 2019 to the Surgical Department of our Hospital with abdominal distension and discomfort in the last 6 months. On physical examination, a palpable large mass was documented in the right hypochondrium extending below the umbilicus with tenderness in the upper abdomen without evidence of ascites. His medical history was little significant, just fraternal twin and hypothyroidism. Laboratory tests were carried out but tumour markers, hepatitis virus markers, and liver enzymes were normal and not diagnostic. Contrast enhanced CT-scan highlighted a giant, hypodense, heterogeneous, solid lesion without cystic component in the right lobe of the liver (Fig. 1). The tumour compressed neighbouring parenchyma and displaced abdominal organs and the retro-hepatic vena cava was also compressed with the middle hepatic vein slightly occluded by thrombotic material. Percutaneous needle biopsies were performed in different areas of the mass. All samples showed mesenchymal tissue with low proliferative index MIB1, and the pathologist suggested diagnosis of MHL. Following a thorough review of the literature, in view of the possibility of malignant degeneration and the high rate of local recurrence, radical liver surgery was proposed. Liver volumetry was measured, and the future liver remnant appeared sufficient to avoid post-operative liver failure. For this reason, liver transplantation was ruled out in favour of right hemi-hepatectomy without segment 1, according to Brisbane classification. In April 2019, the patient was admitted in operatory room after resolution of the middle hepatic vein thrombus with anticoagulant therapy. Right hepatectomy was carried out in 330 min.
The huge right hepatic lobe mobilization was the most difficult surgical step. The hard/elastic consistence of the mass favoured the exposition of the diaphragm. The short hepatic vein was transected and retrohepatic vena cava was mobilized. As soon as vena cava was confirmed free of infiltration, we put a tape in front of the vena, behind to the liver, to facilitate the transection line, carried out with bipolar and CUSA™. We cut the right bile duct away from the biliary main confluence above the caudate process.
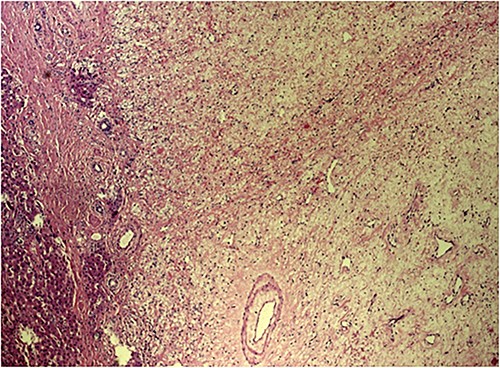
The post-operative course was uneventful, and the patient was discharged on 14th post-operative day. Pathologist’s report described a giant solid tumour without cystic component with maximum diameter of 35 cm, 8 kg weighted (Fig. 3) and clear surgical margins. Microscopic study showed a well circumscribed tumour, surrounded by an irregular margin of compressed hepatic parenchyma. Hypo-cellulated loose myxoid stroma was the almost exclusive component in the centre of the tumour, rich of blood and lymphatic vessels but small bile ducts and hepatocyte islands were nearly absent (Fig. 4). The mesenchymal component of the tumour was reactive to desmin and smooth muscle actin. Low proliferation index was confirmed (MIB1).
In consideration of possible association of liver mesenchymal hamartoma with DICER1 syndrome and Beckwith Wiedemann syndrome, the patient was addressed to a genetic counselling for assessment and indication to genetic testing. First, sequencing analysis of DICER1 and CDKN1C gene was performed with Next Generation Sequencing to detect small intragenic deletion/insertion and missense, nonsense, and splice site variants; it was negative. Second, DNA methylation analysis on the chromosome 11p15 region was performed and it was normal.
At 42 months follow-up liver function tests were normal and CT-scan performed 6 months, 1–2–3 years after surgery were negative for disease recurrence (Fig. 2).
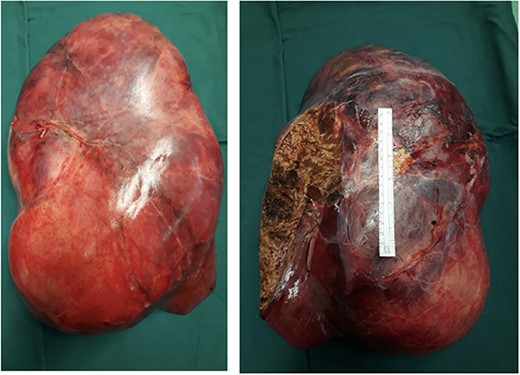
Right and left surface of the tumour. The ruler in the image in a standard 15 cm.
Discussion
Since the first report in 1903 by Maresch, MHL was definitively described only in 1956 by Edmonson. About its aetiology, uncertainties still persist, and some authors consider MHL as ductal plate malformation where the biologic behaviour varies with the relative predominance of blood vessels and bile ducts within the surrounding mesenchymal stroma. Other theories gaining acceptance are regional ischaemia, toxic-metabolic, and a true neoplastic aetiology. Most cytogenetic analysis highlighted chromosomal abnormalities involving the region 19q13.4 which leads to microRNAs dysregulation [8, 9] and other genetic mutations have been described as possible cause of MHL development, linking this kind of mesenchymal tumour to syndromes just like Beckwith-Wiedeman and DICER-1 [10–12].
Grossly, MHL appears as a well-circumscribed, unencapsulated mass, whose size can vary greatly ranging from some centimetres up to >30 cm with a soft, myxoid, and cystic cut surface. They appear as solitary lesion of the right liver lobe but, multifocal cases with satellite nodules have been reported. Microscopically, there is a mixture of epithelial and stromal components where the first is mainly represented by tortuous dilated bile ducts, the second by spindle cells in a background that ranges from myxoid in ~50% of the cases, to collagenous or hyalinized in 47%. Vessels consist of small to medium sized veins or capillaries, hepatocytes are found in cords, islands, or lobules [13–15].
The typical clinical presentation is due to mechanical compression of adjacent viscera causing pain, vomiting, jaundice, poor weight gain, and respiratory distress in huge masses. There are no laboratory tests or tumour markers specific for the diagnosis of MHL. On ultrasound, the classic appearance is of a complex solid-cystic mass with internal septation. On a non-contrast CT, the stromal component appears hypoattenuating while the cystic one is of near water attenuation. The mesenchymal component of these lesions typically enhances with contrast administration. MR imaging of MHL depends on the prevalence of stromal component on the cystic one and generally solid areas appear hypointense to adjacent liver parenchyma both on T1 and T2 weighted images, while cystic ones appear close to water signal intensity on T2 but with a variable signal intensity on T1 depending on protein content of the cysts. At gadolinium administration, enhancement is mild and limited to stromal components and septa [8, 16]. Pre-operative differential diagnosis is complicated also by the possibility that biopsy results sometime might be misleading [17].
While small MHL may spontaneously regress, the current standard of care is complete resection because it has been proven that angiosarcomas and undifferentiated embryonal sarcomas (UES) can arise from MHL not only if left in situ but also several years after incomplete resection [8, 18]. Indeed, coexistence of UES and MHL in the same tumour, similar features on gross pathology, immunohistochemistry and cytogenetics suggest a strong association between these entities and support the theory of possible UES malignant degeneration in MHL. More conservative treatments such as partial resection, cyst drainage, and cyst marsupialization have been linked to complication, recurrence, and poor survival [19]. In case of unresectable MHL, sequential resection can be considered, but for massive tumours, liver transplantation should be an option in order to guarantee a radical treatment [8].
In conclusion, we presented this clinical case because even though extremely rare in adulthood, we believe that clinicians should consider MHL in the differential diagnosis of a liver mass. Their frequently remarkable dimension and the need of radicality impose major liver resections or liver transplantations, which in our opinion should be performed only in referral centres. The knowledge about the origin and the causes of these tumours is still lacking but genetic analysis may represent a fundamental tool to shed light on MHL not only under a pathologic point of view but also for therapeutic purpose.
Conflict of interest statement
None declared.
Funding
The research leading to these results has received funding from UNINI, Università degli Studi di Milano, Via Santa Sofia, 9 Milano, ITALY.
References
- actins
- patient referral
- smooth muscle
- hamartoma
- adult
- chromosomes
- desmin
- differential diagnosis
- dna methylation
- genes
- hepatic resection
- liver transplantation
- mitotic index
- surgical procedures, operative
- abdomen
- diagnosis
- genetic counseling
- liver
- neoplasms
- transplantation
- percutaneous needle biopsy
- mesenchymal hamartoma
- 11p15
- young adult
- excision
- tissue degeneration



