-
PDF
- Split View
-
Views
-
Cite
Cite
Javier A Meza-Hernandez, Asya Zubillaga-Mares, Minnet Serrano-Sanchez, Francisco E Alvarez-Bautista, Mario Trejo-Avila, Alejandra Nuñez-Venzor, An uncommon case of right hepatic duct entering cystic duct associated to multiple complications of biliary tract disease: acute pancreatitis, hepatolithiasis, acute cholecystitis and hepatic abscess, Journal of Surgical Case Reports, Volume 2024, Issue 3, March 2024, rjad637, https://doi.org/10.1093/jscr/rjad637
Close - Share Icon Share
Abstract
Anatomical variations of the biliary tree pose diagnostic and treatment challenges. While most are harmless and often discovered incidentally during procedures, some can lead to clinical issues and biliary complications, making knowledge of these variants crucial to prevent surgical mishaps. Here, we present an unusual and clinically significant case. A 61-year-old man is admitted to the hospital with epigastric pain and diagnosis of pancreatitis of biliary origin and intermediate risk of choledocholithiasis. Magnetic resonance cholangiopancreatography (MRCP) reported hepatolithiasis and choledocholithiasis, whereas endoscopic retrograde cholangiopancreatography showed cystic drain of the right hepatic duct. One month later the patient presented again to the emergency room with increasing abdominal pain and a computed tomography that demonstrated the presence of hepatic abscess and acute cholecystitis. The patient underwent percutaneous drain abscess and a subtotal laparoscopic cholecystectomy. Biliary anatomical variants present challenges on the diagnostic investigations, interventional and surgical procedures, understanding the possible complications is essential.
Introduction
The incidence of extrahepatic biliary tract anatomic variant is reported as high as 47% [1, 2]. Cystic duct (CD) inserted into the right hepatic duct (RHD) has been reported on 0.3 to 2.7% [3–6]. Biliary variants, including those of the CD, can be the direct cause of different diseases. Low insertion of the CD has a stronger association with common hepatic duct (CHD) stone formation, CHD dilatation, and a positive bacterial bile culture than CD with a normal joint with the extrahepatic biliary duct (EHBD) [7]. The anatomical variations of the EHBD are very important during laparoscopic cholecystectomy, living donor liver transplantation, hepatic tumor resection, and therapeutic biliary drainage [8, 9].
We report an extremely rare case of the RHD entering the CD, with multiple clinical presentation including pancreatitis, acute cholecystitis, and hepatolithiasis with a hepatic abscess.
Case presentation
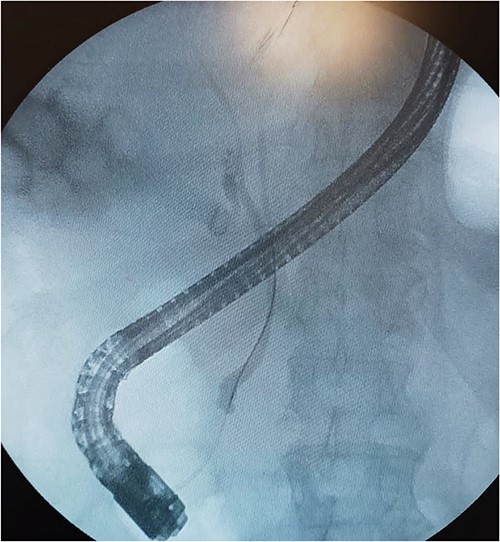
A 61-year-old Mexican man presented to the emergency room with an epigastric pain; he was admitted due to acute pancreatitis of biliary origin and intermediate risk of choledocholithiasis. We performed a magnetic resonance reported, right posterior hepatic duct absence of signal, choledochal with presence of heterogeneous content with absence of signal in intrapancreatic portion of a choledochal duct (Fig. 1). On the endoscopic retrograde cholangiopancreatography (ERCP), a cystic drain of the RHD was found (Fig. 2). Patient presented slow clinical evolution with increased systemic inflammatory response syndrome for which a contrasted computer tomography was performed with findings of pancreatitis with Balthazar C score. Subsequently, an adequate evolution was presented, and the acute picture of pancreatitis is resolved, hospital discharge was performed to an elective laparoscopic cholecystectomy of interval.
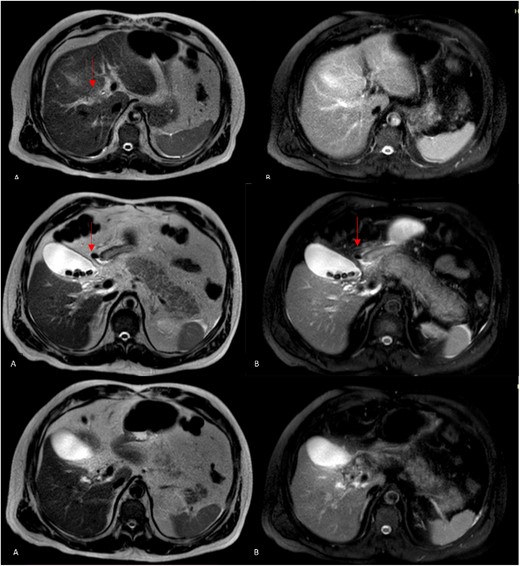
Magnetic resonance cholangiopancreatography (MRCP) axial imagen. A: T2W- TSE; B, T2W- SPIR. A and B findings right posterior hepatic duct absence of signal, choledochal with presence of heterogeneous content with absence of signal in intrapancreatic portion (arrows).
However, 1 month later, the patient had persistent fever and abdominal pain in the epigastric region. He also complained of anorexia, chills, and liquid stools with and temperature on 39°C. The laboratory test reveals a white blood cell count of 26.7× 103/μl, indicating a left shift in neutrophils at 88.3%. The blood biochemistry analysis revealed the following results: total bilirubin, 2.53 mg/dL (normal range 0.30-1.00); direct bilirubin, 1.21 mg/dL (normal range 0.03-0.18); indirect bilirubin, 1.32 mg/dL (normal range 0.27-0.82); albumin, 2.82 (normal range 3.70-5.30); alanine aminotransferase (ALT), 130 IU/L (normal range 7-52); aspartate aminotransferase (AST), 128 IU/L (normal range 13-39); gamma glutamyl transferase (GGT), 263 IU/L (normal range 9-64); alkaline phosphatase, 528 IU/L (normal range 34-104); lactic dehydrogenase (LDH), 214 IU/L (normal range 140-221); amylase, 38 IU/L (normal range 29-103); lipase, 13 IU/L (normal range 11-82); C-reactive protein (CRP), 25.3 mg/dL (normal range 01.000-03.000). We performed a contrasted computer tomography reviewed hepatic abscess (Fig. 3A and B) in segment VIII and V with a volume of 100 mL associated with smaller pericholangiolar abscesses of diffuse distribution, also reported a complete thrombosis of the right branch of the portal vein with partial extension to the main portal vein (Fig. 3D) and free fluid in the pelvic cavity.
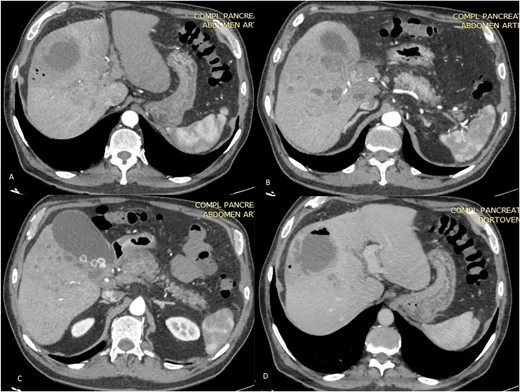
Axial TC on shows liver of regular contours, heterogeneous at the expense of multiple collections that follow the course of the bile duct of diffuse distribution. A and B on different sections: abscess on segments V and VIII, measures 58 × 56 × 67 mm in its major axes and has an approximate volume of 100 cc, presents air-fluid level. The bile duct measures 3.5 mm. C: Distended thin-walled gallbladder with stones inside with an axis >100 mm and calculous cholecystitis. D: (Venous phase). The portal vein is observed with hypodense material inside with partial defect in filling of the contrast agent at the level of the hilum with complete extension toward the right branch.
Therefore, image-guided percutaneous liver collection drainage was performed and 5 days after we performed a laparoscopic cholecystectomy and intraoperatively found a 10 × 5 × 3 cm gallbladder with thickened walls of 0.5 cm (Fig. 4).
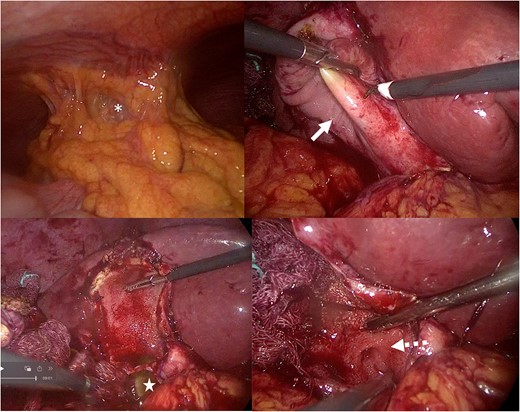
Intraoperative picture: subtotal cholecystectomy fenestrating laparoscopic. A: Inability to visualize the gallbladder due to adhesions (white asterisk): Parkland V. B: Gallbladder (solid arrow) with thickening of the wall. C: Visualization the calculus star). D: The cystic (dotted arrow) was found transversal.
During the procedure, critical view of safety was not available because of a D type cystic insertion of the Benson–Page classification; so, we decided to do a subtotal reconstituting cholecystectomy. After an uneventful postoperative course, the patient was discharged the next day.
Discussion
The anatomy of the biliary tree is notoriously variable [10]. The RHD usually drains the V–VIII segments of the right liver lobe. This is accomplished with two major branches: the right posterior branch draining the posterior segments, VI and VII, and the right anterior duct (RAB) draining the anterior segments, V and VIII [11]. The CD connects the gallbladder to the CHD and typically inserts into the middle third of the EHBD usually on the right side [7]. Normal mid-lateral insertion of CD to extrahepatic bile duct is seen in only 51–72% of population [12].
A systematic review on 2011 described the total number of typical anatomy cases reported was 60.8% and total biliary anatomic variants were 39.2% [13] and another recent systematic review shows the standard anatomy found in 65.7% of the overall population reported [14]. Anatomical variations are anomalies that are asymptomatic in the most of cases. However, some of these variations may predispose to pathologic conditions, such as recurrent pancreatitis, cholangitis, choledocholithiasis, and biliary malignancies [15, 16].
Anatomic factors, including the CD and the hepatic ducts, may present complications and various clinical manifestations that require individualized management [16]. As well constitute one of the major causes of bile duct injuries after laparoscopic cholecystectomy [11] and represents a challenge for the surgeon during the surgical procedure [11].
In Mexico, very few studies have been conducted in relation to the anatomical variations of the biliary tree, Aguirre-Olmedo et al. on 2011 with cholangiography of the ERCP found biliary anatomic variations in 5.1% of patients in the study [17].
There is no standardized classification system to describe biliary anomalies; several classifications have been reported to describe the anatomical variations [18]. Huang et al. classified the right intrahepatic bile ducts depending on the site of insertion of the right posterior sectoral bile duct into five types, this classification is the most utilized system [19]. However, this does not include within the variant insertion of the CD directly on the HRD [20]. On 1921, Walton was the first on described abnormalities of the CD [6]. Benson and Page classified into five main types; they found three proper anatomical variants of CD insertion (Type A, B, and D). Swain et al. [9] on 2020 proposed a classification including the CD variation into nine types (type-A to I) (Fig. 5). In the case of our patient, an extremely rare anatomy variant was observed, which we can classify as variant D, Benson and Page classification [21, 22].
![Benson and Page classification [8, 22]. Type A: a low-lateral insertion with a common sheath enclosing both the CD and common bile duct or a low-medial insertion at or near the ampulla of Vater. Type B: the CD may join the CHD at the porta hepatis, together with the RHD and left hepatic duct, configuring a “trifurcation”. Type C: variant consisting of an accessory hepatic duct. The Type D variant consists of an abnormal insertion of the CD directly into the RHD. Type E variant in which a cholecysto-hepatic duct was present (also known as the duct of Luschka).](https://oupdevcdn.silverchair-staging.com/oup/backfile/Content_public/Journal/jscr/2024/3/10.1093_jscr_rjad637/1/m_rjad637f5.jpeg?Expires=1772505792&Signature=CKpMue0MB2PIqmKS75YFs9ibA1rNQ7xXjbZIRTfZuRjP0iw37oyfc1ZNI31a9ktc-hjsTz-WCC2BgiqtRykUlBl~oxhTafm8PLeT8nslMqz5ZezZZBVcOrF~B-xzrs8OO3D8QVxnluGsYPlVFCJ7eu2Hl4NTHGewYYvIsLtXT-3o-r1dWsQ-UYtqmFiRRpCIpJZvm9vt-hN081TB9RwCPZSYFqAThmhqVDkU-nyNUbHwsqmSZWuYjuZZkFO8Ljhlc1XYPF45m47NoyJpSMQdRxfQgStq2Rx6nhkF81a2TpKaHWnb81HhN7Reul8xVCIaVLfeWSLegWGSFYRpcUc8ow__&Key-Pair-Id=APKAIYYTVHKX7JZB5EAA)
Benson and Page classification [8, 22]. Type A: a low-lateral insertion with a common sheath enclosing both the CD and common bile duct or a low-medial insertion at or near the ampulla of Vater. Type B: the CD may join the CHD at the porta hepatis, together with the RHD and left hepatic duct, configuring a “trifurcation”. Type C: variant consisting of an accessory hepatic duct. The Type D variant consists of an abnormal insertion of the CD directly into the RHD. Type E variant in which a cholecysto-hepatic duct was present (also known as the duct of Luschka).
Several reports suggest that intrahepatic stones are associated with anatomic variation, thus supporting a possible link with these variants, poor bile evacuation, and cholestasis [15, 23, 24]. The presence of variant anatomic could alter the physiological biliary flow and, potentially, cause its stagnation, resulting in the presence of more viscose bile along the entire biliary system [15]. The reduced biliary flow in the EHBD might obstruct or slow down the normal CD outflow, thus leading to abnormal gallbladder emptying and consequent bile stasis in the gallbladder [15].
The theoretic relationship between a type-3 drainage (right posterior sectional duct or right anterior sectional duct; drains directly into the LHD) variant and hepatolithiasis is consider the prevailing theory that biliary stasis and secondary cholangitis may contribute to intrahepatic lithiasis [19]. This is supported by the fact that intrahepatic lithiasis is more common in the left liver because the LHD joins at a more acute angle than the RHD of these patients are theoretically more likely to experience stasis and a greater incidence of intrahepatic lithiasis [19].
Hepatolithiasis is defined as the presence of gallstones in the bile ducts proximal to the confluence of the right and left hepatic ducts, irrespective of the co-existence of gallstones in the common bile duct and/or gallbladder [23]. In Western countries, including Mexico, the incidence is <1% and is generally thought to be secondary to stones originating in the gallbladder or primarily resulting from benign strictures, primary sclerosing cholangitis, choledochal cysts, or malignant biliary tumors [25].
Cholestasis is essential for the formation of intrahepatic stone, both pigment stones and cholesterol stones [23]. The most common pathological changes in intrahepatic stone due to anatomic variants are stones, strictures, dilation, chronic proliferative cholangitis, cholangiocarcinoma, hepatic parenchymal fibrosis, and hepatic atrophy [2, 7]. Triple-phase, contrast-enhanced computed tomography of the abdomen can detect ductal dilatation and stones, precisely define liver anatomy, and identify biliary strictures. While direct cholangiography, which includes procedures such as ERCP or percutaneous transhepatic cholangiography (PTC), stands as the gold standard diagnostic test in hepatolithiasis due to its nearly 100% sensitivity, it is essential to note that this method is invasive and carries a substantial risk of bleeding and acute pancreatitis [26].
Propagation of intrahepatic stones into the extrahepatic biliary tree may cause gallstone pancreatitis, which may be the initial presentation of hepatolithiasis in some patients [26], as is the case of this patient with the first admission for acute pancreatitis. The patient returned to our center due to hepatic abscess as a complication we believe that could be associated to the hepatolithiasis and the anatomic variant.
In this case, image-guided percutaneous liver collection drainage was performed and then subtotal cholecystectomy laparoscopic was performed to avoid injuring the RHD. The hepatic duct may be mistaken for the CD, with potentially bile duct injury [22]. The patient had a good evolution without any complications and the postoperative course was uneventful. As a conclusion, CD variations are important to recognize to improve the management, it is important to have in mind the various clinical manifestations and even the possible complications prior to surgery and during with the goal to avoid them; in our case, completing a timely diagnosis was a challenge, which led the patient to present a liver abscess, we believe that performing the cholecystectomy in a timely manner could avoid reaching these presentations thus reducing morbidity and mortality.
CARE Checklist (2016) statement
The manuscript was prepared and revised according to the CARE Checklist (2016) Statement.
Acknowledgements
To all surgical team at General Hospital “Dr Manuel Gea Gonzalez”.
Author contributions
Javier Andres Meza-Hernández: wrote the paper and contributed data. Asya Zubillaga-Mares: wrote the paper and contributed analysis. Minnet Serrano-Sánchez: contributed data. Alejandra Nuñez-Venzor: designed the study concept and critically revised the manuscript. Francisco E. Alvarez-Bautista: drafted the manuscript. Mario E. Trejo-Avila: critically revised the manuscript.
Conflict of interest statement
None declared.
Funding
The authors have no funding sources to disclose.
References
Gündüz N, Doğan MB, Alacagöz M, et al. . Anatomical variations of cystic duct insertion and their relationship with choledocholithiasis: an MRCP study. Egypt J Radiol Nucl Med 2021;
Rystedt JML, Wiss J, Adolfsson J, Enochsson L, Hallerbäck B, Johansson P, et al. . Routine versus selective intraoperative cholangiography during cholecystectomy: systematic review, meta-analysis and health economic model analysis of iatrogenic bile duct injury. BJS Open. 2021;
- pancreatitis, acute
- cholecystitis, acute
- biliary tract diseases
- choledocholithiasis
- endoscopic retrograde cholangiopancreatography
- hepatic abscess
- abscess
- biliary tract
- cystic duct
- cysts
- surgical procedures, operative
- diagnosis
- laparoscopic cholecystectomy
- magnetic resonance cholangiopancreatography
- right hepatic duct
- liver calculus



