-
PDF
- Split View
-
Views
-
Cite
Cite
Sassi Samia, Khmou Mouna, El M Rihane, Benbella Leila, Hajar Zebbakh, Hatim Essaber, Youssef Omor, Rachida Latib, Mehdi Youssef, El K Bassma, Breast adenomyoepithelioma diagnosed on core needle biopsy: a diagnostic challenge, Journal of Surgical Case Reports, Volume 2024, Issue 2, February 2024, rjae090, https://doi.org/10.1093/jscr/rjae090
Close - Share Icon Share
Abstract
Adenomyoepithelioma represents a rare tumor of the breast characterized by biphasic proliferation of epithelial and myoepithelial cells. Owing to its nonspecific clinical presentation, the rarity, and the morphological pitfalls in differential diagnosis, the diagnosis may be extremely difficult especially on limited samples such as core needle biopsy; thus, the diagnosis is histological, which is confirmed by the specificities of the immunohistochemical analyses. Here we report a case of a 64-year-old female who presented a benign adenomyoepithelioma diagnosed on core needle biopsy, review the clinicopathological features of breast adenomyoepithelioma diagnosed on core needle biopsy, and discuss the useful clues to prompt accurate diagnosis.
Introduction
Adenomyoepithelioma (AME) of the breast is a rare disease characterized by a biphasic proliferation of epithelial and myoepithelial cells. This entity was first described by Hamperl [1]. Most of AMEs of the breast are benign with good prognosis. However, malignant transformation has been reported in some cases [2]. The definite diagnosis requires histopathological examination with immunochemical studies, in order rule out invasive breast carcinoma that represent the main differential diagnosis [3]. Local excision is the gold standard treatment.
The authors describe in this case presentation an AME in 64-year women to review the clinicopathological features of breast AME diagnosed on core needle biopsy, and discuss the useful clues to prompt accurate diagnosis.
Case report
A 64-year-old female with prior history of treated left breast carcinoma, presented to the department of gynecology with a palpable mass inferior-internal quadrant of right breast. Mammography showed a well-circumscribed mass with focally indistinct margins, there were no calcifications (Fig. 1). On ultrasonography, the mass had regular contours with heterogeneous echogenicity, measuring 8 × 5 mm. They were no axillary palpable lymph nodes. Ultrasound-guided core biopsy was performed and the specimen was sent to our laboratory for pathological examination.
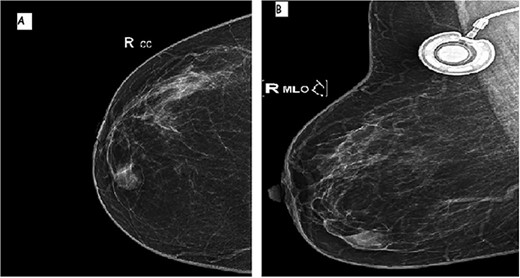
Mammographic image of the right breast, front (A) and side (B) views: inferior-internal quadrant mass of medium density, oval and circumscribed with partially indistinct margins without calcifications.
Histological examination showed a well-circumscribed tumor consisting of glandular structures lined by a double-cell population: regular epithelial cells, with a basal located nucleus without atypia, and myoepithelial cells, with showed large nucleus and fine chromatin with a low nucleo–cytoplasmic ratio. These structures were in bided in dense fibro hyalin stroma (Fig. 2A and B).
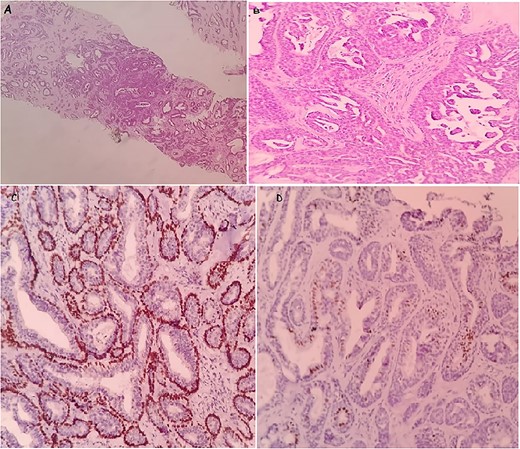
The microscopic images of the case in core needle biopsy: a well-limited tumor proliferation of glandular structures. Hematoxylin and eosin; ×10 (A). Double-cell population, regular epithelial cells and the second, external, corresponds to myoepithelial cells. Hematoxylin and eosin; ×40 (B). Immunohistochemical staining: showing the myoepithelial component was positive for p63 (C) and the epithelial component was positive for ER (D).
Immunohistochemistry confirmed the diagnosis. In fact, the epithelial cells were positive for receptor estrogen (ER) and the myoepithelial cells were positive for p63 and negative for receptor progesterone (RP) (Fig. 2C and D). Thus, the definite diagnosis was AME.
After a follow-up period of 1 year, there was no evidence of recurrence.
Discussion
Breast AME is a rare breast tumor first reported by Hamperl [1]. It is a biphasic neoplasm showing two components: luminal ductal cells and myoepithelial cells. AME is a benign tumor with a low-grade malignant behavior. In fact, given the rarity of AME and the lack of a uniform classification system to date, defining the biological significance of the different patterns of this lesion is difficult; thus, with clinical management is challenging. This tumor has been reported in a female with only two cases reported in man to date [4].
Clinically, AME usually presents as a solitary unilateral palpable mass with variable duration of symptoms (from few weeks to several months). The tumors are usually located in a peripheral portion of the breast, although some lesions were centrally regions [4].
On mammography, AME appears as a round or lobulated, dense, mostly circumscribed mass, sometimes with partially indistinct margins. Atypical findings include: calcifications and cystic appearance. Ultrasound features show solid oval hypoechoic mass with irregular borders. On in MRI imaging as well appears as round, lobulate, or oval masses with clear or shaded borders. Given these variable imaging features, histopathology is a corner stone for the accurate diagnosis.
On gross examination, AME presents as well-circumscribed lobulated white to grayish lesions, ranging from 0.3 to 7 cm. Focal cystic changes, hemorrhage and focal necrosis have been described with irregular borders [5]. Microscopically, it is a well-circumscribed lobulated tumor, surrounded by a fibro-hyaline capsule with expansions. It is composed of well-formed glands, lined by a double layer of internal epithelial and external myoepithelial cells showing neither cytonuclear atypia nor mitotic activity. Myoepithelial cells are polygonal or spindle-shaped cells with clarified or plasmacytoid morphology in some cases. Luminal cells appear as cuboidal with eosinophilic cytoplasm. Four histological patterns of AME have been described: tubular, lobulated, spindled, and adenosis-like; rare variants have been also reported including prominent intraductal papillary pattern [6].
Malignant transformation of tumors has been reported in the literature. Histological features associated with more-aggressive behavior are: high mitotic activity, cytologic atypia with nuclear pleomorphism, prominent nucleoli, hyperchromasia, and necrosis.
Immunohistochemistry is mandatory to confirm the diagnosis, in fact myoepithelial component is positive for: smooth muscle actin, Protein63 and S100 protein; Cytokeratin5/6, calponin while luminal cells are positive for cytokeratin AE1/AE3, epithelial membrane antigen EMA and Estrogen Receptor ER. RP and HER2 are negative in both components.
P53 and KI-67 are known to be prognostic factors in AME; in fact, when they are positive, they yield a poorer outcome [7].
However, the diagnosis of AME on a needle core biopsy can be challenging because of morphologic heterogeneity. In limited biopsy material, the sampled tissue may even be mistaken for invasive carcinoma, especially in tumors that have compact glandular structures with clear cell epithelioid myoepithelial proliferation.
Differential diagnosis of AME is broad, including cellular fibroadenoma, papilloma, phyllodes tumor, myofibroblastoma, breast carcinomas including adenoid cystic carcinoma, metaplastic carcinoma, and extramammary metastases. The most important diagnosis that needs to be ruled out is invasive carcinoma especially in small samples; immunohistochemistry allows for the right diagnosis by highlighting myoepithelial cells [3].
Most AMEs appear to represent variants of intraductal papilloma, the distinction between AME with a papillary architecture and papilloma with prominent myoepithelial cells might be difficult. In fact, MEC hyperplasia in intraductal papilloma is typically focal in contrast to AME, where it is diffuse [8]. Nipple adenoma may mimic AME, useful features to distinguish these two lesions are: the presence of florid ductal hyperplasia, the pseudo infiltrative pattern of stromal sclerosis entrapping glandular epithelium without an entrapped fibrous tissue, and the presence of fibrovascular cores. Clear cell carcinoma and metaplastic carcinoma are also included in the differential diagnosis.
AME are usually benign although they may recur locally. Tubular variants and some lobular tumors with high mitotic activity are particularly prone to local recurrence [9].
The gold standard treatment of AME is surgical excision with clear margins to avoid local recurrence [8]. Our patient had a surgical removal of the tumor; with no recurrence to date (follow-up period of 1 year).
Conclusion
In summary, the pathological diagnosis of breast AME in core needle biopsy is extremely difficult. Pathologists and clinicians need to be aware of as part of the differential for symptomatic and asymptomatic breast masses. The diagnosis of certainty is histological and immunohistochemical with surgical excision is recommended for definitive diagnosis and treatment.
Author contributions
All authors read and approved the final manuscript.
Conflict of interest statement
None declared.
Funding
None declared.
Data availability
Not applicable.
Consent
Informed consent was obtained from the patient.



