-
PDF
- Split View
-
Views
-
Cite
Cite
Luis Palacios-Diaz, Antonio J Aguilar Martínez, Francisco J Pérez Rodríguez, Agustín Penedo, Jose M Sánchez-Márquez, Nicomedes Fernández-Baillo, Total en bloc vertebrectomy and immunochemotherapy for chondrosarcoma colliding with intraosseous lymphoma, Journal of Surgical Case Reports, Volume 2024, Issue 2, February 2024, rjae018, https://doi.org/10.1093/jscr/rjae018
Close - Share Icon Share
Abstract
A 59-year-old woman diagnosed with a Grade I chondrosarcoma in T7 underwent total en bloc vertebrectomy. Analysis of the surgical piece established diagnosis of a Grade 1 chondrosarcoma confined to T7. Surprisingly, an infiltration with diffuse large B-cell lymphoma was found. Systemic disease was ruled out and diagnosis was established as intracompartmental Grade 1 chondrosarcoma colliding with intraosseous extranodal diffuse large B-cell lymphoma. Resection of chondrosarcoma was considered complete and treatment with four cycles of RCHOP was indicated. Two years after surgery, the patient remains at complete metabolic response. To date, this is the first reported case of chondrosarcoma colliding with lymphoma. Although Grade 1 chondrosarcoma is typically managed with local control through complete surgical resection, the mentioned finding of the lymphoma indicated the need for systemic treatment with immunochemotherapy.
Introduction
To date, this is the first reported case of chondrosarcoma coexistent with a lymphoma within a single organ. The case is representative of the multidisciplinary decision-making for complex and opposed therapeutic approaches: surgical resection for Grade I chondrosarcoma and systemic immunochemotherapy for diffuse large B-cell lymphoma (DLBCL).
Case presentation
A 59-year-old woman visited our clinic with Grade I chondrosarcoma diagnosis at T7. She complained about atraumatic dorsal pain and left costal pain, worsened by respiratory movements, with no radiation features or neurological symptoms, reason why she consulted at another clinic. The patient’s father died from esophageal cancer at age 69, her mother developed a colorectal cancer at 71 and died from breast cancer at 80, her sister submitted colectomy because of polyposis and the patient’s 3-year-old-child died from neuroblastoma.
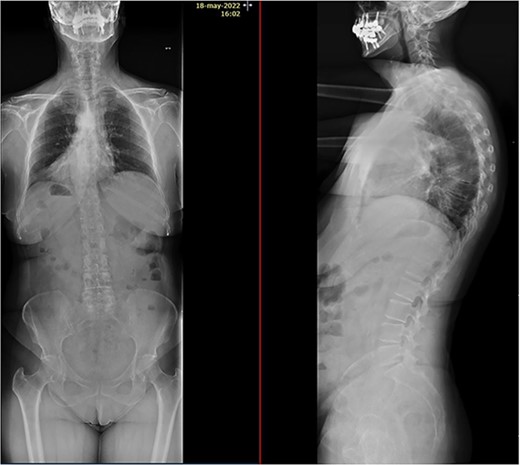
Imaging tests revealed a T7 pathologic fracture (Figs 1 and 2). First, X-ray-assisted percutaneous biopsy and kyphoplasty were inconclusive. Second, CT-assisted percutaneous biopsy and kyphoplasty revealed trabecular infiltration by hypercellular hyaline cartilage with cellular atypia, without relevant mitotic activity. Histological diagnosis of a Grade I chondrosarcoma was then established and the patient was directed to our clinic.
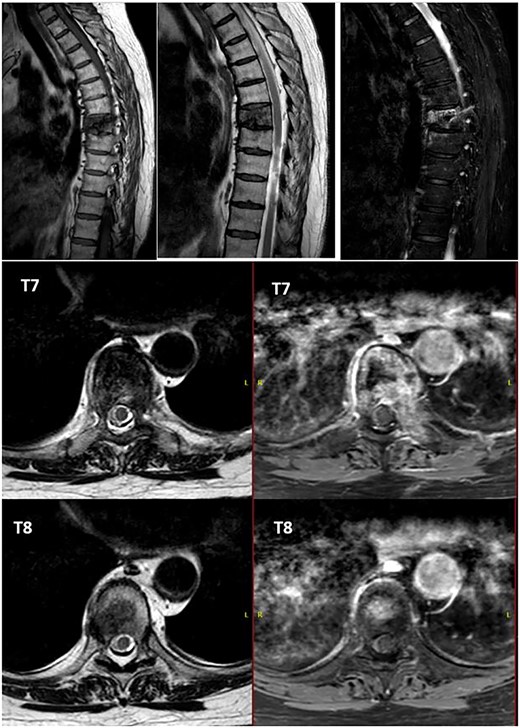
Alteration in the morphology and signal of the T7 vertebral body that presents a fracture with 30% loss of height and dorsal protrusion of posterior wall, with a left and epidural soft tissue component, the signal change extends to the left pedicle and part of the posterior arch. The right lateral margin of T8 vertebral body presents a hypointense signal on T1 and markedly hyperintense on STIR that indicates the presence of osseous edema. These findings suggest T7 pathological fracture and bone edema in the left T8 pedicle.
After PET scan, metastatic disease was discarded. Complete spine magnetic resonance and thoraco-abdominopelvic CT indicated the location on T7 vertebral body, with both end plates broken and causing a leakage to intervertebral disks. Moreover, an intensity change in the left pedicle of T8 was found, which could not be discarded as tumoral (Figs 2 and 3). Surgical resection been the only possible treatment, the patient was appointed to undergo total en bloc vertebrectomy of T7 and T8 and partial of caudal T6, following the technique described by Tomita et al. [1]
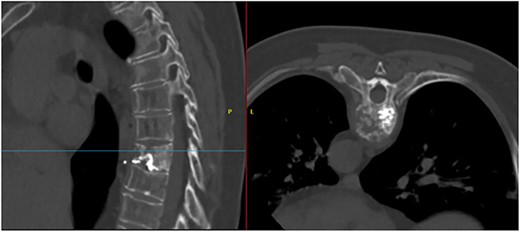
T7 pathological fracture with breakage of both superior and inferior end plates. Cement from the performed kyphoplasty is present, with leakage into the anterior paravertebral space.
Posterior spinal instrumentation was performed with pedicle screws, three levels above and below T7. During the last pedicle coronal passing of the Tomita saw, a complete loss of abdominal and lower limbs potentials happened. Mean blood pressure above 85 mmHg was monitored, patient’s temperature was raised, and NASCIS protocol for acute spinal cord injury was initiated. After coronal cuts of the pedicles were completed, cephalic axial cut at T6 vertebral body and caudal cut at T8T9 intervertebral disk were performed. The piece was then removed en bloc (Fig. 4). Reconstruction with anterior mesh packed with allograft was performed. Posterior reconstruction was completed with double bar on each side for a more stable construct, with proximal tibia autograft covering the laminae for graft support and protection of the posterior spinal cord from muscular pressure (Fig. 5).
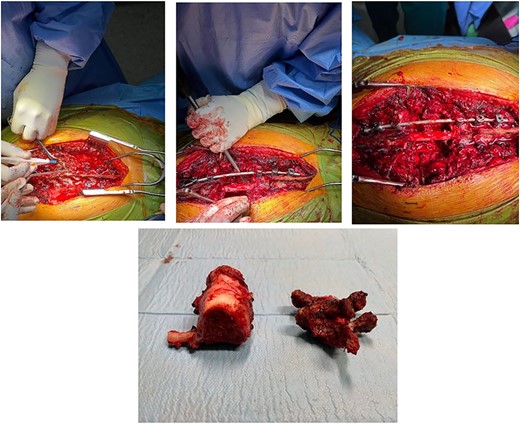
Total en bloc vertebrectomy of T7 and T8 and partial vertebrectomy of caudal T6.
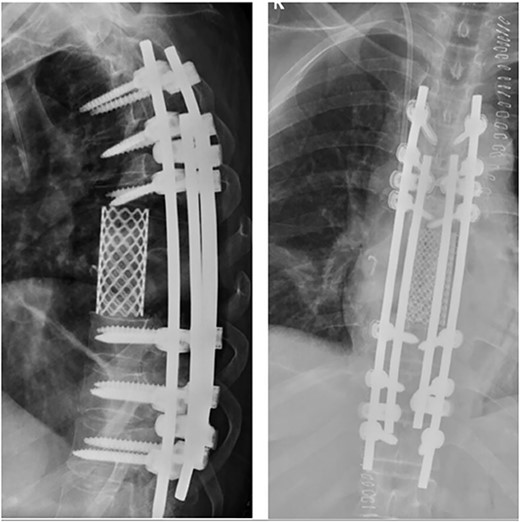
Anterior reconstruction with mesh and posterior reconstruction with T4T5T6, T9T10T11 screws, and double bar.
By the end of surgery, potentials in the left foot recovered with right partial improvement. The patient was admitted postoperatively at rehabilitation to receive treatment over an incomplete motor spinal cord injury (T3-ASIA-D).
Analysis of the surgical piece established diagnosis of well-differentiated chondrosarcoma (G1) confined to T7 without extension to T6, T8, or soft tissues. Surprisingly, infiltration of high-grade neoplastic component of hematolymphoid type was present. Immunohistochemistry was consistent with DLBCL germinal center subtype (vimentin+/CD45+/CD20+/CD10+/BCL-6+/Ki67 > 80%) (Figs 6–8).
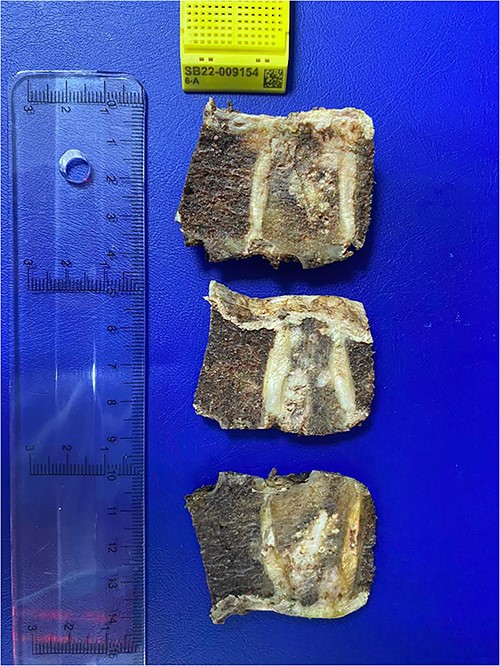
Histopathological exam, macroscopic study. A lobulated lesion (3.8 × 2.6 × 2.9 cm) involves 80% of the central vertebra (T7) and presents cartilaginous features. The lesion is confined to T7. Cavity filled with bone cement is present.
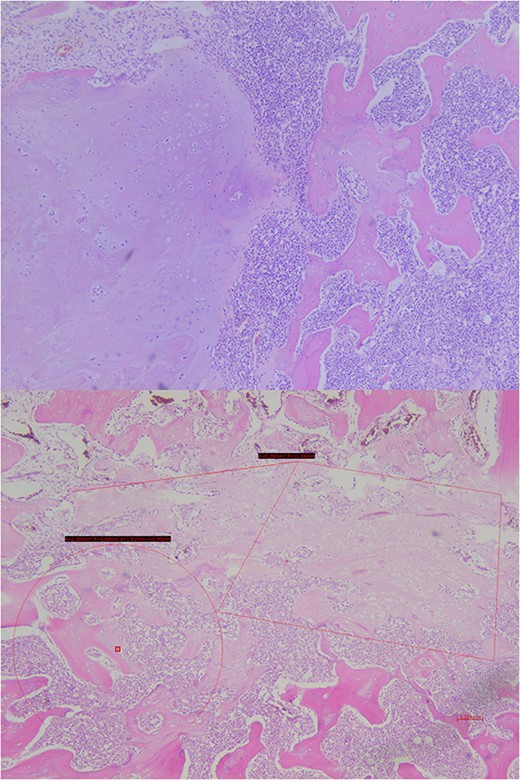
Histopathological exam, microscopic study. The lesion shows trabecular infiltration by hypercellular hyaline cartilage with cellular atypia, with no relevant mitotic activity. This tumor is confined to the central vertebra (D7) and does not extend further (R0). In the central portion, an abrupt transition to a high-grade neoplastic component of hematolymphoid type grows diffusely, composed by medium-large cells, with frequent mitosis. No extension to adjacent soft tissues or to the other vertebrae.
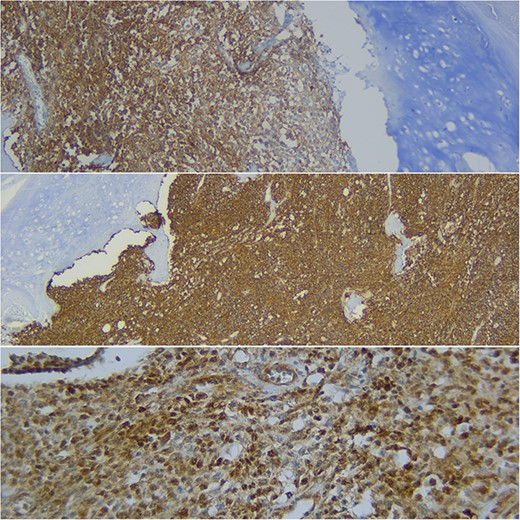
Immunohistochemical study shows how the described hematolymphoid neoplasia presents a immunophenotype typical of B germinal center. CD10+, CD20+, and BCL6+ are showed (from top to bottom).
A second PET was performed and the case was derived to multidisciplinary meeting. Systemic disease was ruled out and diagnosis was established as intracompartmental Grade 1 chondrosarcoma colliding with intraosseous, extranodal DLBCL (Ann Arbor stage IE). In line with the R0 resection, chondrosarcoma was considered to be cured. In regards to DLBCL, immunochemotherapy with RCHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) was indicated by an oncological hematologist. By the end of the fourth cycle, PET showed no evidence of malignant disease, which indicated a complete metabolic response and therefore chemotherapy was discontinued (Fig. 9).
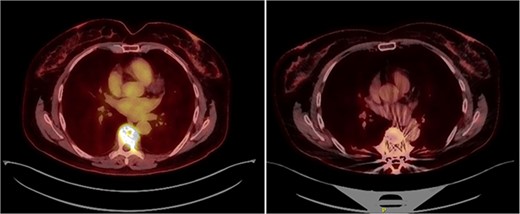
(left) PET scan prior to surgery showing pathological fracture at T7 with increased metabolical uptake (SUVmax 9.48). (right) PET scan by the end of fourth RCHOP cycle. No evidence of malignant disease. Slight metabolical uptake adjacent to spinal instrumentation (SUVmax 3.6) indicative of postsurgical changes.
Two years after surgery, adequate clinical recovery continues, with a high-degree satisfaction, persisting mild lumbar pain, and a slight weakness in the right foot. Pertinent X-rays show normal position of the implants with no implant loosening (Fig. 10).
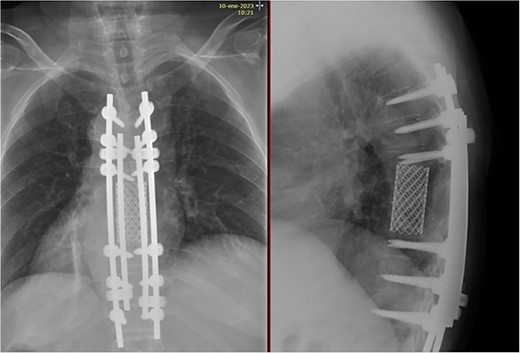
X-rays showing normal position of the implants with no signs of loosening.
Discussion
To date, this is the first reported case of chondrosarcoma colliding with lymphoma within a single organ. Coexistence of these two tumors has been previously reported, although only in distant organs [2].
Genetic predisposition to cancer could be hypothesized, which might consist of a colorectal adenomatous polyposis syndrome associated to chondrosarcoma [3].
Grade 1 chondrosarcomas are considered to have little metastatic potential, rendering en bloc R0 resection the best predictor for overall survival and adjuvant therapies unnecessary after successful surgery [4].
Following the Tomita surgical classification, the tumor would be a Type 3 intracompartmental lesion. Therefore, surgical margin is achievable when the vertebra is cut at the healthy part of the pedicle or lamina. Single posterior approach is preferable in said lesions, as the spinal cord can be carefully observed, especially during anterior column corpectomy. Reconstruction must include an anterior column support combined with posterior spinal instrumentation [1].
The survival rate for spinal chondrosarcomas, especially Grade 1 treated with R0 resection is favorable [5, 6]. However, a high complication rate after multisegmental spinal resections exists, with massive intraoperative bleeding and neurological damage being the most threatening [7–9]. During follow-up, the risk of nonunion is high, especially in the thoracic spine [10]. Therefore, this challenging surgical procedure must be performed by experienced surgeons in close collaboration with a multidisciplinary team.
The singularity of this case relies in the finding of a concomitant tumor with radically opposed treatment: a limited-stage DLBCL (Ann Arbor stage IE) whose frontline treatment is RCHOP immunochemotherapy protocol [11]. In younger patients with favorable prognosis, four cycles of RCHOP are recommended, with no need for consolidation radiotherapy when metabolic response is complete [11, 12]. Disease-free survival in Stage I extranodal DLBCL is also favorable [13].
In this clinical case, given that the resection was complete and initial PET ruled out systemic disease, the need for other treatment aside from surgery was put into question. However, there> is no existing evidence of such therapeutic approach, whereas prognosis in patients with limited-stage DLBCL treated with standard of care immunochemotherapy is excellent.
Conflict of interest statement
None declared.
Funding
None declared.



