-
PDF
- Split View
-
Views
-
Cite
Cite
Paul Brian Ng Hung Shin, Samuel X Tan, Anthony Griffin, Ailin Tan, Vijay Kanagarajah, Cat-scratch disease masquerading as post-transplant lymphoproliferative disorder, Journal of Surgical Case Reports, Volume 2024, Issue 2, February 2024, rjad223, https://doi.org/10.1093/jscr/rjad223
Close - Share Icon Share
Abstract
Lymphadenopathy in an immunosuppressed patient raises the quintessential diagnostic dilemma: infection or malignancy? We present the case of a transplant recipient on anti-rejection prophylaxis admitted with acute fever, malaise and a swollen right axillary node. The patient had pancytopenia and tested positive for Epstein–Barr virus; nodal core biopsy demonstrated atypical plasma cell infiltration, immediately raising suspicion for post-transplant lymphoproliferative disorder. However, excisional biopsy and Bartonella henselae serology clarified a final diagnosis of cat-scratch disease—a potentially fatal zoonosis requiring a disparate treatment regimen. Here, we explore this patient’s investigations, hospital course and recovery, with an emphasis on recognizing and differentiating these diagnostic mimics in post-transplant practice.
Introduction
Cat-scratch disease (CSD) is a vector-borne infection caused by the gramme-negative bacterium Bartonella henselae [1]. In immunocompetent individuals, CSD typically presents as a regional lymphadenopathy associated with mild pyrexia and malaise [2]. However, B. henselae has a heightened incidence of dissemination in immunocompromised patients, and CSD demands brisk antimicrobial therapy in this cohort because of the corresponding risk of bacteraemia, bacillary angiomatosis and peliosis [3].
In contrast, post-transplant lymphoproliferative disorder (PTLD) is a neoplastic condition classically involving uncontrolled proliferation of Epstein–Barr virus (EBV)-infected B cells in the absence of T-cell surveillance [4]. PTLD also presents with lymphadenopathy, pyrexia and malaise; however, unlike CSD, PTLD is treated by ceasing immunosuppression, with consideration of rituximab and chemotherapy. To illustrate the pitfalls of distinguishing CSD and PTLD in this patient group, we report a case of a renal transplant patient with investigative findings initially suggestive of transplant-associated malignancy.
Case report
A man in his early 50s was admitted to a tertiary hospital with a 10-day history of fever, rigours, headache, fatigue and generalized arthralgia most prominent in the bilateral proximal fingers. He denied recent travel, physical trauma, occupational exposures, new sexual partners or sick contacts. Physical examination identified a tender, firm and mobile mass in the right axilla, ~2 cm in diameter, that the patient had not previously noticed.
His medical background was significant for a renal transplant 6 years prior in the setting of a congenital kidney condition. Maintenance immunosuppression was achieved with prednisolone, tacrolimus and mycophenolate sodium. We additionally note that the patient was a dog owner who reported no known contact with cats in the 6 months preceding admission.
On admission, baseline investigations included a urine microbiology, blood cultures, full blood count, Chemistry screen −20, C-reactive protein (CRP) and a chest X-ray. Subsequently, throughout the inpatient stay, a battery of tests was organized. These included nasopharyngeal respiratory virus polymerase chain reaction (PCR; SARS-CoV-2, Influenza A/B, Respiratory Syncytial Virus, Parainfluenza 1 to 4, Adenovirus, Human Metapneumovirus, Rhinovirus), Mycoplasma pneumonia Tot Ab, Legionella longbeachae 1 TotAb and Legionella pneumophila 1/2 TotAb, Parvovirus B19 PCR. The infective screen further broadened to test for hepatitis A, B, C, HIV, Bartonella, Brucella, Leptospirosis, Cryptococcus, Syphyllis, Toxoplasma Gondii, Strongyloides and Polyoma virus. Additionally, a lumbar puncture was performed to test for bacterial and viral aetiology—HSV1/2; Varicella zoster, CMV and EBV. Moreover, the patient also had an ultrasound of his axilla, a CT chest/abdomen/pelvis, a transthoracic echocardiogram to assess for endocarditis as well as a fluorodeoxyglucose (FDG)-positron emission tomography (PET) scan.
Initial blood-work revealed pancytopenia, with a haemoglobin of 116 g/L, white cell count of 3.2 × 109/L and platelet count of 103 × 109/L. Serum uric acid (11.9 mg/dL), lactate dehydrogenase (LDH; 938 units/L) and CRP (56 mg/L) were markedly elevated. The patient tested positive for EBV on PCR) blood testing, suggesting reactivation in the context of a primary EBV infection in 2007. Routine infective screening was otherwise negative, including respiratory serology and PCR; blood serology and PCR; and cultures for blood, urine, stool and cerebrospinal fluid. Notably, serum B. henselae IgG (enzyme immunoassay) was negative (<1: 64; cut-off 1: 256) on admission.
Ultrasound of the right axillary mass indicated a single enlarged lymph node measuring 26 × 25 × 40 mm (Fig. 1). Following discussion with haematology and infectious disease specialists, a nodal core biopsy was performed. This identified an atypical lymphoid infiltrate primarily characterized by CD138+/IRF4+ plasma cell clusters, interspersed by CD3+/CD5+ T cells and scattered necrotic foci (Fig. 2). As this was a core biopsy, nodal architecture was not appreciable. There was mild positivity for plasma cells on EBV-encoded small RNA in situ hybridization (EBER-ISH), consistent with the patient’s positive EBV PCR status. Bacterial and fungal specimen culture was negative, including for Mycobacterium tuberculosis, and flow cytometry did not detect any monotypic B cells or aberrant T cells.
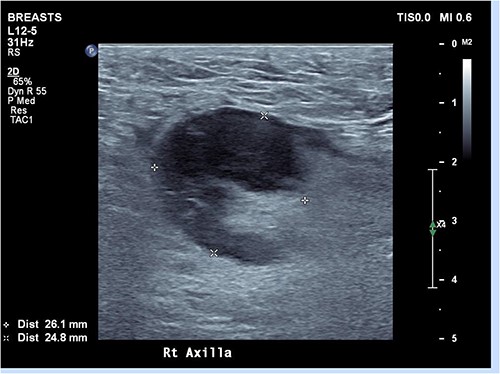
Ultrasound of right axilla showing an enlarged right axillary lymph node. This node retained its fatty hilum but had thickened cortex, measuring up to 13 mm in short axis.
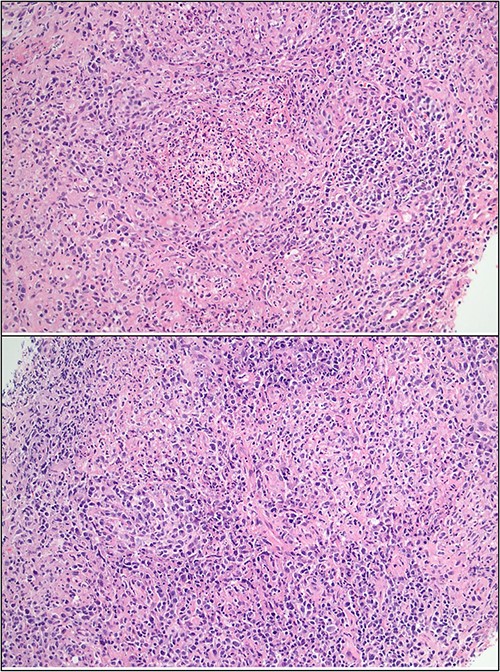
Core biopsy histology showing lymphoid infiltrate with foci of suppuration and dense plasma infiltrate.
Given the histologic finding of an EBER-ISH+ lymphoid population coupled with pancytopenia, hyperuricaemia and elevated serum LDH, we favoured a provisional diagnosis of polymorphic PTLD. This was seemingly validated by PET scanning, which demonstrated FDG avidity in multiple right axillary lymph nodes (Fig. 3).
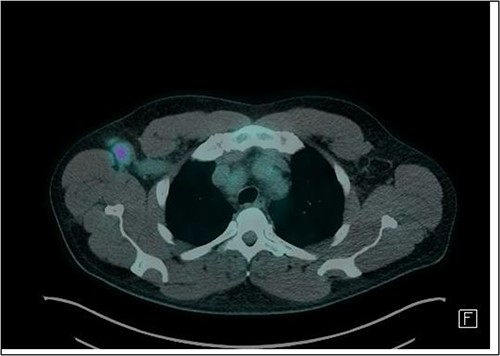
Axial slice of FDG-PET CT scan showing a 22 mm (short-axis) moderately FDG-avid right axillary lymph node (SUV-max 4.19).
Additional PCR testing for B. henselae and Toxoplasma gondii was conducted on the core biopsy specimen, and B. henselae DNA was detected. To investigate the possibility of comorbid PTLD, excisional node biopsy was performed. Histology demonstrated histiocytic granulomas with a surrounding lymphoid infiltrate; crucially, there was no effacement of nodal architecture on the full specimen (Fig. 4). At this stage, serum B. henselae IgG was repeated and returned a positive result (>1: 1024; cut-off 1: 256), indicating seroconversion within the 15-day period from his negative B. henselae IgG at admission.
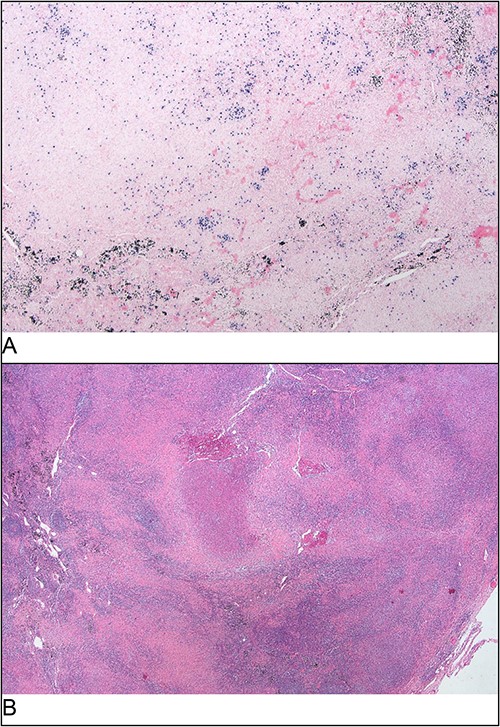
Excisional biopsy histology (A) foci of necrotising granulomatous inflammation; (B) presence of EBER-ish-positive cells.
The patient was commenced on oral azithromycin at 500 mg daily and achieved full resolution of his symptoms while in hospital. Following discharge, he continued oral azithromycin for a total of 3 months. In light of his sustained clinical recovery, no changes were made to his regular immunosuppressive therapy during or after admission.
At 6 months following his initial admission, the patient remained well. He denied further symptoms; however, he continued to have a palpable right axillary node on examination. His full blood count panel and serum LDH normalized though his serum uric acid was modestly elevated at 8.7 mg/dL. He will continue to be followed up through our transplant and haematology outpatient clinics.
Discussion
The immune system is a cardinal safeguard against both exogenous pathogens and endogenous neoplasia [5]. In neutralizing the immune response to graft tissue, immunosuppressive agents simultaneously predispose transplant recipients to opportunistic infections and de novo malignancies, including CSD and PTLD [6]. Both CSD and PTLD clinically present with lymphadenopathy and a nonspecific inflammatory syndrome. Histologically, both conditions can present with a lymphoid infiltrate and associated necrosis: CSD as an uncommon response to B. henselae antigen presentation [7], and PTLD because of uptake of viral exosomes and expression of oncogenic EBV proteins [6].
On our initial infective screen, only EBV returned positive, which highlighted the need to consider EBV-associated disorders. This was corroborated by the finding of an EBER-ISH+ plasma cell-dominated infiltrate with necrotic features on core biopsy, however in conjunction with the patient’s blood-work and PET scan raised the critical differential of PTLD. Tissue PCR and repeat serum IgG for B. henselae returned a positive result, confirming the alternative diagnosis of CSD. A confounder was that CSD is classically transmitted through feline saliva, and the patient firmly denied any recent contact with cats. However, there is evidence that canines are also capable of transmitting CSD [8] and we thus presume that the patient’s pet dog represented the causative exposure.
Several authors have previously commented on the diagnostic similarities between CSD and PTLD [9–11]. However, there is sparse literature on how to resolve this ambiguity in practice. Notably, Boyle et al. [10] identified that transplant-associated Bartonellosis is often confounded by an incidentally positive EBV PCR, in concordance with our case. Similarly, as ~20% of PTLDs are EBV-negative, a negative PCR does not necessarily exclude malignancy—though it should be noted that the majority of EBV-negative PTLDs are T-cell driven [12]. FDG-PET is generally sensitive and specific at distinguishing PTLD from non-malignant disease [6], and can simultaneously exclude diffuse or multi-organ disease. However, we noted that CSD-associated lymphadenopathy has also the potential to be highly FDG-avid [9], further compounding this diagnostic quandary.
While we remained wary of concomitant PTLD, there were two reassuring factors in this patient’s case. First, PTLD-associated lymphocytes characteristically degrade the underlying tissue [4]; therefore, the finding of wholly preserved nodal architecture on excisional biopsy considerably reduces suspicion for polymorphic PTLD. Second, the patient’s EBV load was negative on discharge despite uninterrupted immunosuppressive therapy.
Serum B. henselae IgG and EBV PCR are the first-line screening tests for CSD and PTLD, respectively. Bartonella henselae IgG is moderately sensitive for CSD, but as a plasma cell product will struggle to identify acute disease [13]. In contrast, B. henselae IgM testing is not routinely offered in Australia because of the relatively brief period of IgM production and correspondingly low sensitivity [13, 14]. EBV DNAemia can support a differential of PTLD; however, as 20–30% of the population will incidentally test positive for EBV on blood PCR, quantification of viral load is important [15]. Both assays can be repeated 2–4 weeks after the initial test to observe trends and, in the case of CSD, allow for greater IgG production [6, 13]. If a core biopsy is sampled, B. henselae PCR and EBER-ISH should be conducted on the tissue specimen.
The gold standard for delineating CSD from PTLD is a histological examination of an excisional biopsy, allowing evaluation of the nodal architecture. Malignant PTLD infiltrate almost invariably results in nodal effacement; in contrast, preserved nodal architecture is suggestive of CSD or early stage hyperplasia [6]. Though not evidenced in this case, necrotising granulomas offer a further histologic clue: as an indicator of impaired pathogen clearance, they are a common feature in CSD [7] but absent in PTLD.
Disseminated B-henselae and untreated PLTD can both be fatal. Distinguishing between these conditions is a priority. We aim to emphasize several key investigative considerations to reduce the likelihood of a missed diagnosis.
Conclusion
CSD and PTLD are diagnostic mimics and considering one should raise suspicion for the other. Initial B. henselae serology and EBV PCR results may be misleading. While FDG-PET scanning and core biopsy are essential investigations for PTLD, excisional biopsy may be required to confirm a diagnosis. Bartonella henselae PCR and EBER-ISH should be conducted on the biopsy specimen, and repeat blood testing can be performed after 2–4 weeks if the diagnosis remains unclear.
Conflict of interest statement
None declared.
Funding
None declared.
Data availability
Data is available upon request.



