-
PDF
- Split View
-
Views
-
Cite
Cite
Mutlu Unver, Suleyman Cağlar Ertekin, Eyüp Kebapcı, Mustafa Olmez, Erhan Ergin, Safak Ozturk, Erkan Sahin, Ragıp Ortac, Differential diagnosis of submucosal gastric tumors: gastric schwannomas misdiagnosed as GISTs, Journal of Surgical Case Reports, Volume 2024, Issue 12, December 2024, rjae793, https://doi.org/10.1093/jscr/rjae793
Close - Share Icon Share
Abstract
Schwannomas commonly occur in the head and neck region but are rarely seen in the gastrointestinal tract; the stomach and small intestine are the most commonly involved sites. These tumors are usually misdiagnosed as gastrointestinal stromal tumors (GISTs) before histopathological confirmation due to radiological similarity. GI schwannomas show positivity for S100 protein and vimentin but are negative for CD 117 and CD 34, which helps in differentiating the tumor from GISTs. Case 1: a 70-year-old woman was referred to our hospital by complaints of abdominal pain and discomfort. Upper GI endoscopy demonstrated a protruding lesion at the lesser curvature of the gastric body, and fine-needle aspiration biopsy showed chronic inflammation without malignancy. Since the lesion was suspected to be GIST, this patient had surgery, and a gastric schwannoma was resected successfully. Case 2: a 66-year-old female with anemia and abdominal discomfort was found to have a submucosal elevated mass at the greater curvature of the antrum. Fine needle aspiration biopsy was suggestive of a spindle cell tumor resembling GIST. The patient underwent subtotal gastrectomy with Roux-en-Y reconstruction. Histopathology confirmed schwannoma. It is necessary to differentiate gastric schwannomas from other submucosal tumors of the stomach, especially GISTs. Surgical complete resection of schwannomas usually has a good prognosis with a low probability of recurrence. Though rare, gastric schwannomas should be included in the differential diagnosis of submucosal gastric tumors because the correct identification of this tumor type helps in proper management and evasion of unnecessary extensive surgery.
Introduction
Gastrointestinal schwannomas (GS) are rare neoplasms. They comprise ~3% of all mesenchymal tumors of the gastrointestinal (GI) tract and represent ~0.2% of GI malignancies [1, 2]. They originate from Auerbach’s plexus or, less frequently, from Meissner’s plexus [3]. Though they generally take place in the head and neck region, GS are also found in the GI tract, where they most frequently occur in the stomach and the small intestine [4]. Most GS are asymptomatic, found incidentally, and appear as slow-growing, encapsulated benign tumors with very small malignant potential [5, 6]. GS are commonly mistaken for GISTs on radiological presentation. Immunohistochemically, GS stain for S100 protein and vimentin but remain negative for CD 117 and CD 34, opposite to what is observed in GISTs [7]. Complete surgical resection gives an excellent prognosis. We present two GS cases initially misdiagnosed as GIST.
Case 1
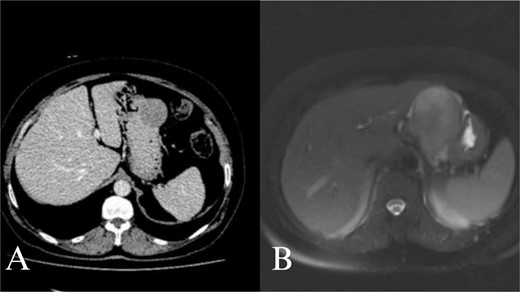
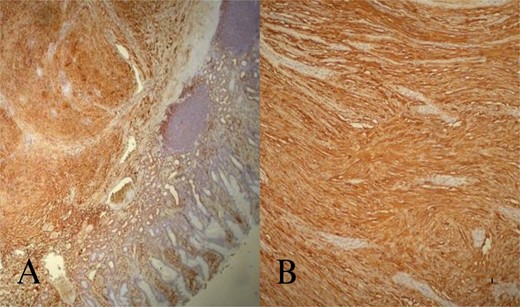
A 70-year-old female was referred to our hospital because of a 6-month history of abdominal discomfort and epigastralgia. Admission vitals, physical exam, routine blood tests, and tumor markers showed normal results. Upper GI endoscopy revealed one protruding lesion 8 cm in size at the lesser curvature of the gastric body. Fine needle aspiration showed chronic inflammation without malignancy. Computed tomography (CT) of the upper abdomen showed a mass originating from the greater curvature of the stomach (Fig. 1A). With suspected GIST, we proceeded with surgery. At laparotomy, an 8 × 7 × 7 cm mass was found at the greater curvature and resected en-bloc with gastric wedge resection. Microscopically, the tumor consisted of spindle cells, positive for S-100 protein and vimentin, while negative for CD 117, CD 34, β-catenin, SMA, synaptophysin, chromogranin, and desmin, consistent with schwannoma (Fig. 2A and B). Postoperative recovery was smooth, and the patient was discharged on Day 5. At 2 years, the patient remains healthy and recurrence-free.
Microscopic examination of the tumor. Tumor composed of spindle cells in the submucosa (HE; ×40). S100 positive tumor cells at immunhistochemical staining (DAB; ×100).
Abdominal CT and MRI images. Contrast enhanced axial tomography revealed a hipodens mass with smooth boundary located at the greater curvature of the stomach. Axial T1 weighted MRI revealed a hypointense, moderate contrast enhancing mass with smooth boundary located at the gastric antrum.
Case 2
A 66-year-old female with a history of abdominal discomfort, epigastralgia, and anemia for 10 months was admitted. The results of admission showed good vital status and general condition except for a low level of hemoglobin at 7.0 g/dL and hematocrit at 23.1%. Upper GI endoscopy showed a submucosal mass at the greater curvature of the antrum. Biopsy cytology demonstrated spindle cell morphology and thus suspected GIST. CT and magnetic resonance imaging (MRI) revealed the mass at the gastric antrum with smooth-bounded margins without lymphadenopathy (Fig. 2B). Laparotomy showed a mass measuring 6 × 6 × 7 cm. En-bloc excision of the mass had been done with subtotal gastrectomy and Roux-en-Y reconstruction. This was histologically proven through positive S-100 and vimentin markers that are negative for CD 117, CD 34, and other markers and confirmative of schwannoma. She did well post-operatively and was discharged on Day 7. She remains well at 18 months follow-up with no recurrence.
Discussion
Schwannomas, also named neurilemmomas, are normally benign neurogenic tumors arising from Schwann cells and may develop anywhere along the peripheral course of nerve [4, 8]. Schwannomas usually develop in the head and neck, but they can rarely develop in the GI tract. The most common site for GI tract schwannomas is the stomach. GS represent ~3% of all mesenchymal tumors of GI tract and ~0.2% of all of the GI tract tumors [1, 2]. They usually develop in the fifth or sixth decade with a female predominance [9]. GS are usually asymptomatic and usually discovered incidentally at laparotomy or radiographically [10]. Upper GI bleeding and abdominal pain are the most common presentations in GS [11].
The main differential diagnosis for GS is a GIST [8]. It is always very difficult to discriminate a GS from a GIST, so schwannomas are often misdiagnosed as GISTs on radiological examinations [2, 12]. It is necessary to discriminate GS from other gastric submucosal tumors, especially from GISTs. Although similar small GISTs and GS may have findings on imaging in common, GS more frequently exhibit exophytic or mixed growth patterns, a homogeneous enhancement pattern, perilesional lymph nodes, and slower growth compared to GISTs [13]. GS are usually well-defined, rounded mural masses with homogeneous attenuation and tend to lack cystic change and hemorrhage on CT scan. Typically, GS are sharply demarcated, strongly enhancing tumors, having low to medium signal intensity on T1-weighted images and high signal intensity on T2-weighted images.
Endoscopic sonography (EUS) has been considered the most valuable imaging modality in the diagnosis of GS. Heterogeneous hypoechogenicity or isoechogenicity, a well-demarcated margin, fourth-layer origination, and lack of cystic change and calcification may be considered helpful findings for the diagnosis of GS on EUS evaluation [2]. Despite EUS has been considered the best modality in the diagnosis of GS, the recent advances in sonographic technology have permitted us to apply transabdominal sonography as a useful method in the assessment of GS [14]. The diagnostic accuracy of EUS-guided fine needle aspiration biopsy (EUS-FNA) was reported as 52% and 55% for EUS-guided Trucut biopsy (EUS-TCB) in mediastinal and retroperitoneal schwannomas. But, GS diagnosed by endoscopic biopsies was reported rarely [15].
The final diagnosis of GS often requires pathological and immunohistochemical examination of surgical specimens. Immunohistochemically, GS are positive staining for S-100 protein and vimentin and negative staining for c-KIT, actin, desmin, and CD34 [1]. In contrast to the GISTs, malignant schwannomas are very rare. Histopathologically, malignant schwannomas have higher cell proliferation, abdominal mitosis, cellular atypia, and invasion. In advanced malignant GS, the tumor could metastasize to the liver and disseminate to the peritonea, but tumors do not metastasize to the lymph nodes [1].
So, the prognosis for GS is usually very good after complete resection. Until now, recurrence of disease has been noted only after incomplete resection [3, 8, 9]. So with the suspicion or diagnosis of GS, complete margin-negative surgical resection should be performed. Routine long-term follow-up should therefore not be offered, and the operation should be considered curative unless any signs of malignant transformation are identified [15].
Conclusion
Gastric schwannomas are rare, benign mesenchymal tumors that must be differentiated from other submucosal stomach tumors, particularly GISTs, requiring complete surgical resection.
Author contributions
All authors have read and approved the manuscript. Mutlu Unver, Suleyman Cağlar Ertekin, Eyüp Kebapcı, Mustafa Olmez, Erhan Ergin, Safak Ozturk, Erkan Sahin, Ragıp Ortac: data collection, revision, and editing; Mutlu Unver, Suleyman Cağlar Ertekin, Erhan Ergin, Safak Ozturk: conceptualization, writing & revision; Mutlu Unver, Suleyman Cağlar Ertekin, Erkan Sahin: preparation and editing of the radiology part; Mutlu Unver, Suleyman Cağlar Ertekin, Ragıp Ortac: preparation and editing of the pathology part.
Conflict of interest statement
All authors declare they have no conflict of interest.
Funding
No funding was received.
Ethics approval
Written consent to publish potentially identifying information, such as details or the case and photographs, was obtained from the patient.
Consent for publication
The patients signed written consent for the surgical procedure. Consent for publication is available.
Guarantor
Suleyman Cağlar Ertekin (the corresponding author).



