-
PDF
- Split View
-
Views
-
Cite
Cite
Hongyang Deng, Xiaodong Xu, An improvement of pancreaticojejunostomy: double-row and six-suture method pancreaticojejunostomy for pancreaticoduodenectomy, Journal of Surgical Case Reports, Volume 2024, Issue 12, December 2024, rjae772, https://doi.org/10.1093/jscr/rjae772
Close - Share Icon Share
Abstract
With the rapid development of pancreaticoduodenectomy (PD) surgery, the incidence of postoperative pancreatic fistula (POPF) and surgical complications has been greatly reduced. The occurrence of POPF is closely related to the quality of the pancreatic reconstruction. Pancreaticojejunostomy (PJ) remains a significant technical challenge, and no PJ has yet been widely recognized. From January 2021 to December 2023, 72 patients underwent PD with double-row and six-suture PJ. The clinical characteristics and postoperative outcomes of these patients were analysed. The median operation time was 240 min (180–540 min). The median intraoperative blood loss was 200 ml (50–600 mL). The postoperative hospital stay was 11 days (8–27 days). Eleven patients (15.3%) had a biochemical fistula and five patients (6.9%) had a grade B POPF. No patient had a grade C POPF or died within 90 days after surgery. Double-row and six-suture PJ is a safe and acceptable PJ.
Introduction
Pancreaticoduodenectomy (PD) is one of the most common operations in abdominal surgery. With the advancement of techniques and the concept of perioperative management, as well as the improvement of surgical skills, the mortality rate for PD decreased significantly from an initial 50% to <5% in the last two decades [1]. However, postoperative pancreatic fistula (POPF) remains a focus topic in pancreatic surgery. As reported, the incidence of grade B/C POPF was ~10% to 25% [2]. POPF is always the main factor leading to delayed hospital stay, complications, unplanned reoperation, and even death. In general, the mortality caused by POPF is high ~30% [3]. Therefore, the strategy of POPF prevention still warranted more attention in PD.
The occurrence of POPF is related to multiple factors such as the texture of the pancreas, the diameter of the main pancreatic duct (MPD), obesity, surgeon’s proficiency, and the pancreatic reconstruction technique [4]. The selection of a suitable pancreaticojejunostomy (PJ) is undoubtedly very important. Despite decades of development, the exploration and choice of the best PJ method remain inconclusive. Therefore, a PJ method with feasibility and clinical practicability, especially, would effectively reduce the incidence of POPF, which still needs to be constantly explored.
The Blumgart anastomotic (BA) was first described by Blumgart in 2000, which contains the four to six U-sutures technique [5]. As a classic end-to-side duct-to-mucosa PJ, it was widely accepted and modified by many surgeons around the world. However, we thought that this PJ was still deficient and improved this technique for PD, which is called double-row and six-suture PJ. Here, we present the details of this PJ and the clinical results.
Materials and methods
Patients
Between January 2021 and December 2023, 72 patients who underwent double-row and six-suture PJ for PD at the second department of general surgery of the Second Hospital of Lanzhou University were enrolled in this study. All PJ procedures in the cases included in the study were performed by the same surgeon. The clinical data of the included patients were collected from the information department of the Second Hospital of Lanzhou University. The basic patient information we collected included age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, preoperative biliary drainage, expansion of the pancreatic duct, etc. The outcome measures focused on in this study included operative blood loss, operation time, POPF (grade B or C), mortality, postoperative hospital stay, etc. This study was exempted by the Ethics Committee of the Second Hospital of Lanzhou University (approval number: 2019A-287). Written informed consents were obtained from all patients for this study.
The method of double-row and six-suture PJ
After resection of the lesion, the stump of the pancreas was electrocoagulated to seal the small pancreatic duct in the section. Meanwhile, we found the MPD. Four transverse U-sutures (4–0 prolene suture) were performed on the upper and lower edges of the pancreas and the upper and lower edges of the MPD, respectively. Each needle entry point was selected about 1 cm from the stump of the pancreas surface. After the suture of the full thickness of the posterior wall of the jejunum, the sutures were again pierced at about 0.5 cm from the previous entry point near the pancreatic section. Then two other transverse U-sutures (4–0 prolene suture) were performed ~1 cm behind the midpoints of the two front-row U-shaped sutures of the two sides of the MPD (Figs 1A and 2A). The threads were not knotted. A 3–5 cm long stent tube, made by the intravenous infusion tube, was inserted into the MPD (Fig. 2B). An incision was cauterized with an electric knife at the location of the jejunum that corresponds to the MPD. Four stitches were intermittently sutured between the MPD and the jejunal mucosa (Fig. 1B). As the MPD and jejunal mucosa were anastomosed, the supporting tube was inserted into the reserved hole of the jejunal (Fig. 2C). The six pancreatic sutures were sutured to the anterior wall of the jejunum again (Fig. 1C). Finally, the six sutures were tightened so that the pancreas was attached to the jejunal serous membrane (Figs 1D and 2D).
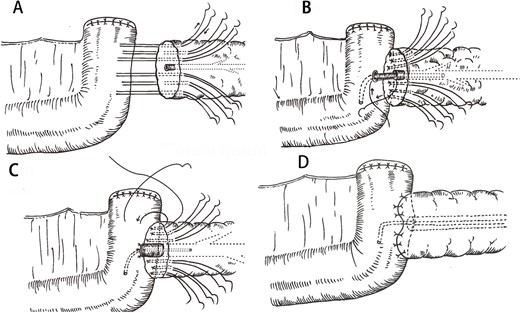
Key process of the double-row and six-suture PJ. (A) Four U-sutures were distributed at the upper and lower margins of the pancreas and the upper and lower of the MPD. Two back-row U-sutures were designed about 1 cm posterior to the midpoints of the two front-row U-suture points of the two sides of the MPD. (B) A support tube was placed in the MPD and four stitches were intermittently sutured between the MPD and the jejunal mucosa. (C) Six U-sutures were again passed through the anterior wall of the jejunum. (D) Finally, the pancreas and jejunum were tied together.
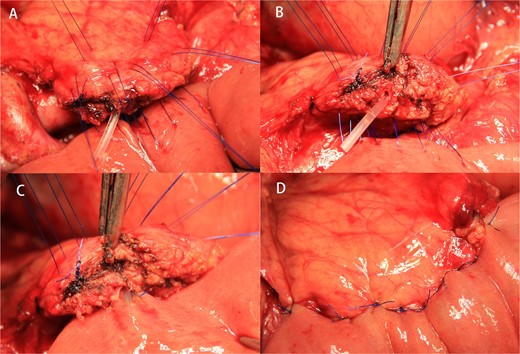
The double-row and six-suture PJ were shown in an operative scene. (A) The layout of double-row and six-suture on the pancreas. (B) An unsecured stent tube was placed in the MPD. (C) Four interrupted sutures were placed between the MPD and the jejunal mucosa under the guidance of the stent tube. (D) Six sutures were tightened after passing through the anterior wall of the jejunum again.
Follow-up and statistical analysis
The patients were followed up by telephone 6 months after discharge. Mortality was calculated only within 90 days after surgery. Postoperative hospital stay was defined as the number of days from the day of surgery to the day of discharge. Measurement data was expressed as medians and ranges. Count data was expressed as n (%).
Results
Among the 72 patients, 47 were men and 25 were women. The median age was 62 years (range 33–79 years). The BMI was 15.3–28.6 kg/m2 (median, 22.4 kg/m2). Thirty patients (41.7%) underwent preoperative biliary drainage due to severe obstructive jaundice. There were 25 cases (34.7%) of pancreatic head carcinoma, 27 cases (37.5%) of duodenal adenocarcinoma, 12 cases (16.7%) of distal bile duct carcinoma, and 8 cases (11.1%) of ampullary carcinoma (Table 1).
| Sex (male/female) | 47/25 |
| Age (years) | 62 (33–79) |
| BMI (kg/m2) | 22.4 (15.3–28.6) |
| ASA score, n (%) | |
| I-II | 62 (86.1%) |
| III-IV | 10 (13.9%) |
| Smoking history, n (%) | 23 (31.9%) |
| Drinking history, n (%) | 16 (22.2%) |
| Diabetes, n (%) | 14 (19.4%) |
| Coronary heart disease, n (%) | 4 (5.6%) |
| Preoperative biliary drainage, n (%) | 30 (41.7%) |
| Previous Abdominal/Pelvic Surgery, n (%) | 14 (19.4%) |
| Jaundice, n (%) | 30 (41.7%) |
| Abdominal pain, n (%) | 32 (44.4%) |
| Preoperative ALB (g/L) | 38.2 (34.1–41.8) |
| Preoperative CA19–9 (U/mL) | 24.6 (12.3–127.2) |
| Location of the tumor, n (%) | |
| Pancreatic head, n (%) | 25 (34.7%) |
| Duodenum, n (%) | 27 (37.5%) |
| Distal bile duct, n (%) | 12 (16.7%) |
| Ampulla, n (%) | 8 (11.1%) |
| Sex (male/female) | 47/25 |
| Age (years) | 62 (33–79) |
| BMI (kg/m2) | 22.4 (15.3–28.6) |
| ASA score, n (%) | |
| I-II | 62 (86.1%) |
| III-IV | 10 (13.9%) |
| Smoking history, n (%) | 23 (31.9%) |
| Drinking history, n (%) | 16 (22.2%) |
| Diabetes, n (%) | 14 (19.4%) |
| Coronary heart disease, n (%) | 4 (5.6%) |
| Preoperative biliary drainage, n (%) | 30 (41.7%) |
| Previous Abdominal/Pelvic Surgery, n (%) | 14 (19.4%) |
| Jaundice, n (%) | 30 (41.7%) |
| Abdominal pain, n (%) | 32 (44.4%) |
| Preoperative ALB (g/L) | 38.2 (34.1–41.8) |
| Preoperative CA19–9 (U/mL) | 24.6 (12.3–127.2) |
| Location of the tumor, n (%) | |
| Pancreatic head, n (%) | 25 (34.7%) |
| Duodenum, n (%) | 27 (37.5%) |
| Distal bile duct, n (%) | 12 (16.7%) |
| Ampulla, n (%) | 8 (11.1%) |
Abbreviations: BMI: body mass index, ASA: American Society of Anesthesiologists, ALB: albumin.
| Sex (male/female) | 47/25 |
| Age (years) | 62 (33–79) |
| BMI (kg/m2) | 22.4 (15.3–28.6) |
| ASA score, n (%) | |
| I-II | 62 (86.1%) |
| III-IV | 10 (13.9%) |
| Smoking history, n (%) | 23 (31.9%) |
| Drinking history, n (%) | 16 (22.2%) |
| Diabetes, n (%) | 14 (19.4%) |
| Coronary heart disease, n (%) | 4 (5.6%) |
| Preoperative biliary drainage, n (%) | 30 (41.7%) |
| Previous Abdominal/Pelvic Surgery, n (%) | 14 (19.4%) |
| Jaundice, n (%) | 30 (41.7%) |
| Abdominal pain, n (%) | 32 (44.4%) |
| Preoperative ALB (g/L) | 38.2 (34.1–41.8) |
| Preoperative CA19–9 (U/mL) | 24.6 (12.3–127.2) |
| Location of the tumor, n (%) | |
| Pancreatic head, n (%) | 25 (34.7%) |
| Duodenum, n (%) | 27 (37.5%) |
| Distal bile duct, n (%) | 12 (16.7%) |
| Ampulla, n (%) | 8 (11.1%) |
| Sex (male/female) | 47/25 |
| Age (years) | 62 (33–79) |
| BMI (kg/m2) | 22.4 (15.3–28.6) |
| ASA score, n (%) | |
| I-II | 62 (86.1%) |
| III-IV | 10 (13.9%) |
| Smoking history, n (%) | 23 (31.9%) |
| Drinking history, n (%) | 16 (22.2%) |
| Diabetes, n (%) | 14 (19.4%) |
| Coronary heart disease, n (%) | 4 (5.6%) |
| Preoperative biliary drainage, n (%) | 30 (41.7%) |
| Previous Abdominal/Pelvic Surgery, n (%) | 14 (19.4%) |
| Jaundice, n (%) | 30 (41.7%) |
| Abdominal pain, n (%) | 32 (44.4%) |
| Preoperative ALB (g/L) | 38.2 (34.1–41.8) |
| Preoperative CA19–9 (U/mL) | 24.6 (12.3–127.2) |
| Location of the tumor, n (%) | |
| Pancreatic head, n (%) | 25 (34.7%) |
| Duodenum, n (%) | 27 (37.5%) |
| Distal bile duct, n (%) | 12 (16.7%) |
| Ampulla, n (%) | 8 (11.1%) |
Abbreviations: BMI: body mass index, ASA: American Society of Anesthesiologists, ALB: albumin.
The median operation time was 240 min (180–540 min). The median intraoperative blood loss was 200 ml (50–600 ml). Intraoperative blood transfusion was performed in 4 cases (5.6%). The pancreatic parenchyma was soft in 53 cases (73.6%). The preoperative computed tomography examination showed dilation of MPD (≥3 mm) in 56 patients (77.8%). The incidence of POPF [6], postoperative hemorrhage [7], and delayed gastric emptying [8] was evaluated according to the definitions of the International Study Group of Pancreatic Surgery. Eleven patients (15.3%) had a biochemical leak and five patients (6.9%) had grade B pancreatic fistula. Delayed gastric emptying occurred in five patients (6.9%). Grade A postoperative hemorrhage occurred in 1 patient (1.4%). The median postoperative hospital stay was 11 days (8–27 days). Intraabdominal infection occurred in seven patients (9.7%), of which one patient (1.4%) underwent reoperation due to intestinal fistula, but no patient underwent reoperation due to POPF (Table 2).
| Operation time (min) | 240 (180–540) |
| Dilated MPD (≥3 mm), n (%) | 56 (77.8%) |
| Pancreatic texture (soft), n (%) | 53 (73.6%) |
| Intraoperative blood loss (mL) | 200 (50–600) |
| Intraoperative blood transfusion, n (%) | 4 (5.6%) |
| Intraabdominal infection, n (%) | 7 (9.7%) |
| Delayed gastric emptying, n (%) | 5 (6.9%) |
| Postoperative hemorrhage, n (%) | 1 (1.4%) |
| POPF | |
| Biochemical leak, n (%) | 11 (15.3%) |
| Grade B, n (%) | 5 (6.9%) |
| Grade C, n (%) | 0 |
| Reoperation, n (%) | 1 (1.4%) |
| Postoperative hospital stay (days) | 11 (8–27) |
| 90-day mortality, n (%) | 0 |
| Operation time (min) | 240 (180–540) |
| Dilated MPD (≥3 mm), n (%) | 56 (77.8%) |
| Pancreatic texture (soft), n (%) | 53 (73.6%) |
| Intraoperative blood loss (mL) | 200 (50–600) |
| Intraoperative blood transfusion, n (%) | 4 (5.6%) |
| Intraabdominal infection, n (%) | 7 (9.7%) |
| Delayed gastric emptying, n (%) | 5 (6.9%) |
| Postoperative hemorrhage, n (%) | 1 (1.4%) |
| POPF | |
| Biochemical leak, n (%) | 11 (15.3%) |
| Grade B, n (%) | 5 (6.9%) |
| Grade C, n (%) | 0 |
| Reoperation, n (%) | 1 (1.4%) |
| Postoperative hospital stay (days) | 11 (8–27) |
| 90-day mortality, n (%) | 0 |
Abbreviations: MPD: main pancreatic duct. POPF: postoperative pancreatic fistula.
| Operation time (min) | 240 (180–540) |
| Dilated MPD (≥3 mm), n (%) | 56 (77.8%) |
| Pancreatic texture (soft), n (%) | 53 (73.6%) |
| Intraoperative blood loss (mL) | 200 (50–600) |
| Intraoperative blood transfusion, n (%) | 4 (5.6%) |
| Intraabdominal infection, n (%) | 7 (9.7%) |
| Delayed gastric emptying, n (%) | 5 (6.9%) |
| Postoperative hemorrhage, n (%) | 1 (1.4%) |
| POPF | |
| Biochemical leak, n (%) | 11 (15.3%) |
| Grade B, n (%) | 5 (6.9%) |
| Grade C, n (%) | 0 |
| Reoperation, n (%) | 1 (1.4%) |
| Postoperative hospital stay (days) | 11 (8–27) |
| 90-day mortality, n (%) | 0 |
| Operation time (min) | 240 (180–540) |
| Dilated MPD (≥3 mm), n (%) | 56 (77.8%) |
| Pancreatic texture (soft), n (%) | 53 (73.6%) |
| Intraoperative blood loss (mL) | 200 (50–600) |
| Intraoperative blood transfusion, n (%) | 4 (5.6%) |
| Intraabdominal infection, n (%) | 7 (9.7%) |
| Delayed gastric emptying, n (%) | 5 (6.9%) |
| Postoperative hemorrhage, n (%) | 1 (1.4%) |
| POPF | |
| Biochemical leak, n (%) | 11 (15.3%) |
| Grade B, n (%) | 5 (6.9%) |
| Grade C, n (%) | 0 |
| Reoperation, n (%) | 1 (1.4%) |
| Postoperative hospital stay (days) | 11 (8–27) |
| 90-day mortality, n (%) | 0 |
Abbreviations: MPD: main pancreatic duct. POPF: postoperative pancreatic fistula.
Discussion
Despite decades of development of PD, POPF, as one of the most harmful complications, often leads to postoperative hemorrhage, infection, and shock [9]. Among the many risk factors for POPF, surgical technique, diameter of MPD, and pancreatic texture are undoubtedly crucial [4]. The texture of the soft pancreas is vulnerable to damage, both from surgical violence and suture cutting, and the damage is exacerbated during intestinal peristaltic recovery. If the diameter of MPD is small, it will make it difficult for MPD processing, such as difficult to suture, easy to tear the pancreatic duct, and easy to narrow.
To minimize the occurrence of POPF, several key points related to PJ should be considered under the guidance of available evidence [10]. We must ensure that the pancreatic juice is discharged without obstruction. Ensuring blood flow to the pancreatic jejunal anastomotic site can prevent anastomotic necrosis. It is also important to minimize damage to the pancreas by sutures, such as pinhole leakage and suture-cutting force. Ensure that the wall of the jejunum is in close contact with the cut pancreatic surface without excessive tension.
The BA applying transpancreatic, full-thickness, mattress U-sutures is supposed to reduce tangential tension and shear force [11]. BA reported only 1.6% mortality and 6.9% grade B/C POPF in 2010 [5]. Fujii reported a simplified BA in 2014 [12]. They thought that too many stitches would cause pinhole pancreatic leakage, so he reduced the original 4–6 stitches to 1–3. The suture line of BA was too dense at the stump of the pancreas and the suture line was less after Fujii et al. reduced the suture line, which was easy to produce the cutting effect. Therefore, we further improved BA and named it double-row and six-suture PJ.
The six stitches of double-row and six-suture PJ followed the U-suture of BA, which evenly shared the tension between the pancreatic and the wall of the jejunum, minimizing the cutting effect of the suture on the pancreas. Therefore, this technique could minimize disruption of the soft pancreas from the cutting effect of the sutures. However, the six sutures were designed to be four in the front row and two in the back row, which solved the problem of anastomotic ischemia caused by overcrowding of sutures as much as possible. At the same time, two triangular structures formed above and below the MPD, thus enhancing the stability of the pancreatoenteric anastomosis. In terms of the MPD, we placed the support tube to prevent the stenosis of the MPD. What’s more, the support tube could guide us to suture the MPD to the jejunal mucosa, which could reduce the difficulty of suturing the small MPD. Furthermore, the unsecured support tube would fall into the jejunum after intestinal function is restored, and no implants would be left at the anastomotic site. Overall, patients who underwent double-row and six-suture PJ showed only 15.3% biochemical leak and 6.9% grade B POPF, indicating that our PJ is safe and acceptable. We thought that double-row and six-suture PJ is suitable for patients with soft pancreas and small MPD.
This study also has limitations in that it was a single-center sample study and lacked a control group. Double-row and six-suture PJ were not attempted laparoscopically due to their complexity, but we thought it may be suitable for robotic pancreaticoduodenectomy.
Conclusion
In this study, we reported on the technique details and clinical results of double-row and six-suture PJ. Our data suggested that this PJ technique is safe and acceptable. However, large samples of randomized controlled trials are needed to further verify our results.
Conflict of interest statement
The authors declare that they have no competing interests.
Funding
Cuiying Scientific and Technological Innovation Program of the Second Hospital of Lanzhou University (CY2019-BJ02).
Data availability
The data and materials are available from the corresponding authors upon reasonable request.



