-
PDF
- Split View
-
Views
-
Cite
Cite
C Charalampous, E Kofopoulos-Lymperis, A Pikouli, P Lykoudis, N Pararas, D Papaconstantinou, C Nastos, D Myoteri, D Dellaportas, Gastric conduit reconstruction after esophagectomy with right gastroepiploic artery absence: a case report, Journal of Surgical Case Reports, Volume 2023, Issue 8, August 2023, rjad474, https://doi.org/10.1093/jscr/rjad474
Close - Share Icon Share
Abstract
Gastric conduit reconstruction is the standard choice after esophagectomy. Conduit’s vascular supply is of primary importance mainly based on right gastroepiploic vessels. A 57-year-old male with absent right gastroepiploic artery, due to a duodenal bleeding ulcer treated with gastroduodenal artery ligation 10 years ago, was treated for gastroesophageal cancer and required esophagectomy. Surgical merits of this troublesome scenario are highlighted. Previous surgical history is highly important for patients requiring complex surgery as esophagectomy. The use of the stomach as conduit after esophagectomy is always the primary option; however vascular supply of it should not be compromised. Variations are rare and careful planning may overcome obstacles as in this case.
INTRODUCTION
Using the stomach toward recreating the anatomical substitute of the esophagus after esophagectomy is the standard conduit choice due to stomach’s easy access, vascularity and plasticity. The right gastroepiploic artery (RGEA) is crucial for blood supply maintenance to the stomach, considered essential for this kind of reconstructive option [1]. Adequate blood supply to the replacement organ is the key to a successful operation [2]. In this case report a patient with absent RGEA who underwent transthoracic esophagectomy using a gastric conduit to restore gastrointestinal continuity is presented along with controversies faced in such a clinical scenario.
CASE PRESENTATION
A 57-year-old white male reached for medical advice after 15 months of progressive dysphagia and weight loss. The patient had a history of ‘kissing’ duodenal ulcers (DU) which caused perforation with concomitant posterior DU bleeding 10 years ago; at the time he underwent exploratory laparotomy and gastroduodenal artery (GDA) ligation as well as ‘Graham patch’ procedure for the perforated DU. A few months later the patient developed pyloric stenosis and gastric outlet obstruction, for which he was treated with re-exploration and gastrojejunal anastomosis-bypass (Fig. 1). Clinical symptoms of dysphagia, regurgitation and reflux led to esophagogastroduodenoscopy 2 months prior to admission, which revealed esophageal lumen stenosis, Barrett’s esophagus and a sizeable mixed type hiatus hernia. Endoscopically acquired biopsy confirmed high-grade dysplasia on the background of Barrett’s esophagus, progressing to invasive esophageal adenocarcinoma. His past medical history was significant for a 4.4 cm infrarenal abdominal aorta aneurysm and hypertension. The patient was admitted to the hospital for nutritional support and further preoperative evaluation. Contrast-enhanced computed tomography (CT) angiography performed to evaluate vasculature of the stomach revealed absence of the RGEA as expected since GDA was ligated in his previous operation (Fig. 2).After complete staging and multidisciplinary meeting discussion the decision was to proceed with primary surgery. Histopathology exam revealed a gastroesophageal adenocarcinoma staged as pT3N1 [3].
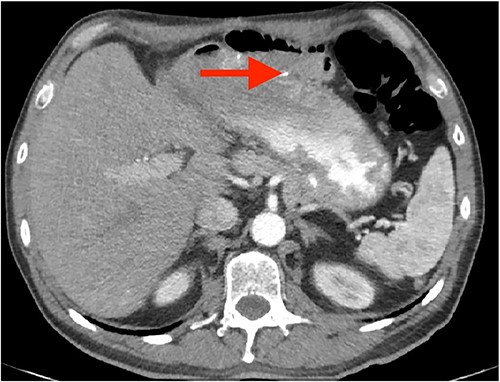
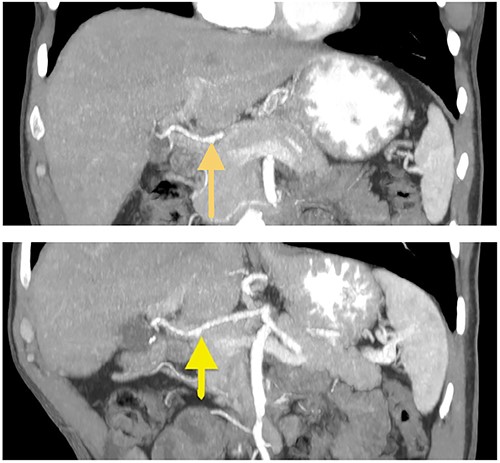
Coronal CT images of stomach’s vascular supply (arrows showing RGEA absence).
DISCUSSION
Gastrointestinal continuity restoration after esophagectomy is generally accomplished using a gastric tube or the entire stomach if available [4]. The success of this standard surgery primarily depends on the RGEA as well as the RGA [5]. The challenge imposed by this particular case was whether the RGA alone could ensure reconstruction viability. The RGEA absence was not new or due to an inadvertent intraoperative injury of this vessel, but a long-standing condition which has probably meant collateral vascularity from the remaining gastric vessels has developed over the years [6]. In addition, the greater curvature gastrojejunostomy might also have provided another route of vascular collateral supply to the gastric remnant after resection of the lesser curvature of the stomach, along with left gastric vessels division. Moreover, the left thoracoabdominal approach made possible to use a shorter gastric conduit in comparison with an Ivor-Lewis or a McKeown procedure, and the esophagogastric anastomosis was performed as low as possible to achieve clear esophageal proximal margin, and oncological adequacy. Lower chest anastomosis meant shorter gastric conduit and probably less worrisome vascular supply of the anastomotic gastric area [7].
Arguments against using the stomach as conduit in the above described clinical scenario would be the high risk of postoperative anastomotic dehiscence or late structuring of the esophagogastric anastomosis. The alternative would be to use large bowel as conduit, from which it is well known that functional results are inferior to gastric conduit use [8]. In addition, prior belief that the RGEA is the sole necessary and irreplaceable vessel for the stomach’s viability was proven false [9]. The results of the method above described are coherent with similar cases involving arterial branch unavailability as reported by Lin et al. [10], who also successfully managed to create a viable gastric conduit in the grounds of gastroepiploic artery absence due to prior medical interventions. Moreover, Masakazu et al. [11] also reported the management of a similar case with missing RGEA due to prior pseudoaneurysm embolization.
To the best of our knowledge this is the first reported case of successful esophageal reconstruction with gastric conduit in the grounds of both RGEA and GDA unavailability to treat a patient with esophageal carcinoma. Preoperative assessment with contrast enhanced CT angiogram is advised to be conducted to delineate blood supply of the stomach, in patients with a history of abdominal surgery potentially compromising gastric vessels. As extensively explained in an interesting article by Maglangit et al. [12] the use of Indocyanine Green (ICG) fluorescence angiography is a powerful tool combined with common surgical intraoperative evaluation of gastric conduit’s viability toward assuring adequate vascular perfusion. Ongoing clinical trials of the ICG utilizations may lead to a breakthrough in the field of esophageal replacement options [13, 14]. Certain limitations arise with the restricted availability of the method.
CONCLUSION
Successful esophagectomy was conducted along with gastric remnant reconstruction despite the absence of the RGEA and the GDA. This case report is focused on pointing out that long-standing absence of RGEA might not be an absolute contraindication for using the stomach as a conduit for esophagectomy due to collateral networks ensuring the required blood perfusion. Preoperative evaluation and left thoracoabdominal approach when feasible are advised in this unusual clinical context.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest regarding publication of this article.
FUNDING
None.
DATA AVAILABILITY
Previously reported case reports were used to support this study and are available and cited at relevant places within the text as references.
CONSENT FOR PUBLICATION
Written informed consent for publication of this manuscript and any accompanying images were obtained from the patient.



