-
PDF
- Split View
-
Views
-
Cite
Cite
Claire K Foley, Marybeth S Hughes, Charles T Hehman, Malignant primary melanoma of the colon: a case report, Journal of Surgical Case Reports, Volume 2023, Issue 8, August 2023, rjad469, https://doi.org/10.1093/jscr/rjad469
Close - Share Icon Share
Abstract
Melanoma is most associated with cancer of the skin. However, a small subset of these melanomas can be a primary malignancy of other mucosal membranes. A 55-year-old male presented to the gastroenterologist with 1 year of symptoms typical of colon cancer including bloating, abdominal pain and weight loss. He underwent colonoscopy and a mass was seen in the transverse colon that was later proven melanoma. A PET CT scan showed this was his only focus of disease. He then underwent a laparoscopic-assisted extended right hemicolectomy. He had an uneventful postoperative course. He was thoroughly examined for other sources of melanoma such as cutaneous, anal and uveal sources. He has recovered well at home and is receiving adjuvant pembrolizumab immunotherapy. Mucosal primary melanomas have a worse 5-year survival than primary cutaneous melanomas. A multi-disciplinary approach is necessary to treat and properly diagnose these malignancies.
INTRODUCTION
Melanoma most commonly occurs cutaneously. Increased sun exposure is a well-known risk factor; however, ~1–4% of primary melanomas in the United States occur within mucous membranes that receive virtually no sun exposure such as the gastrointestinal (GI), respiratory and genitourinary tracts [1, 2]. When a tumor in the gastrointestinal tract is suspected to be a melanoma, further investigation is warranted to determine if it is a primary or metastatic lesion as the vast majority of melanoma in the GI tract is metastatic [2]. Melanoma results in more metastatic lesions to the GI tract than any other cancer [3]. When trying to rule out a primary melanoma in a different location, the diagnostic workup should include gathering a detailed history, as well as an extensive physical exam with emphasis on the dermatologic, ophthalmic and genitourinary exams.
Primary GI melanomas are rare, making up about 2% of all mucosal melanomas [1]. Among primary GI melanomas, distribution of lesions favors mucosal membranes with melanocytes native to that area with tumors identified in the pharynx (32.8%), anal canal (31.4%), rectum (22.2%), esophagus (5.9%), stomach (2.7%), small bowel (2.3%), gallbladder (1.4%) and colon (0.9%) [4]. Primary colonic melanoma is an exceptionally rare diagnosis and as of 2018, only 36 cases of primary colonic melanoma have been reported [2, 5–7]. Among the reported primary colonic melanomas, the ascending colon (46.7%) and cecum (26.7%) were the most common locations [2, 5]. We present a case of primary colonic melanoma in a 55-year-old male who presented to an academic center for definitive resection.
CASE PRESENTATION
Fifty-five-year-old African American male presents to his primary care doctor. He has been experiencing 1 year of symptoms including abdominal boating, cramping and abdominal pain. He has also had some intermittent constipation for this time. He also had noticed that his bowel movements had become more loose and smaller in caliber. He had never had a colonoscopy. His primary care physician recommended a Cologuard test, which came back as positive. His medical and surgical history is unremarkable with the exception of a 40 pack-year smoking history.
He was then referred to gastroenterology and underwent diagnostic colonoscopy that showed a large polypoid mass in the transverse colon that was partially obstructing. Gastroenterology then obtained a CT scan of the chest, abdomen and pelvis that showed wall thickening of the colon (Figs 1–3). Ultimately when pathology confirmed malignant melanoma, a PET scan was ordered that showed increased uptake at the previously seen and biopsied transverse colon mass (Fig. 4).
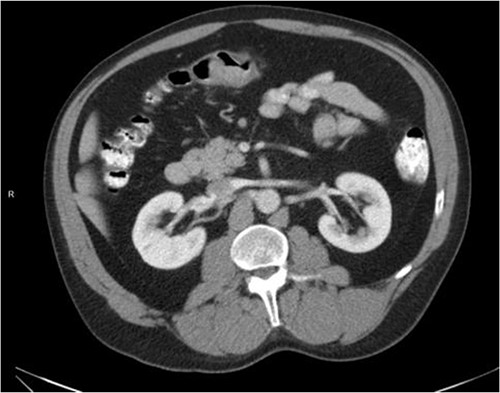
Axial CT scan with IV contrast showing thickened transverse colon.
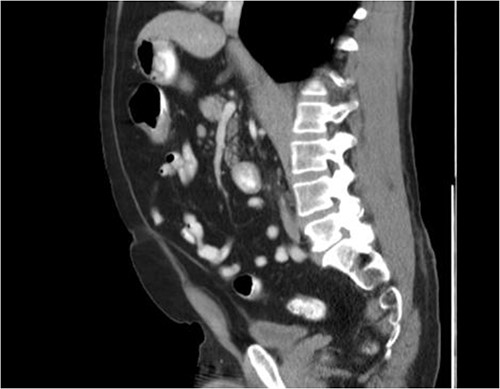
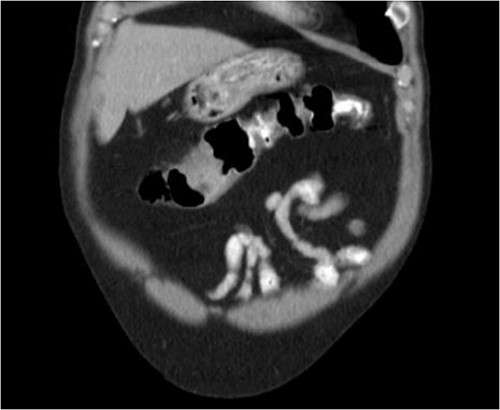
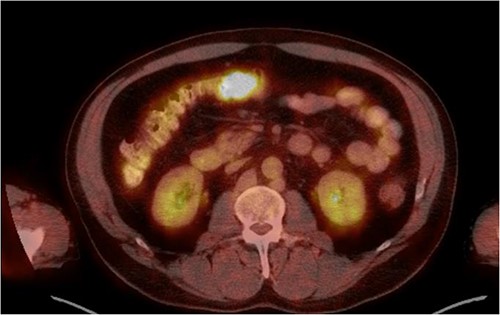
The pathology of this mass was primary colonic melanoma. He then underwent laparoscopic-assisted extended right hemicolectomy. Postoperatively, he went to the medical-surgical floor for recovery. His diet was slowly advanced and his pain was controlled. He was discharged home with return of bowel function and tolerating regular diet on postoperative day 3.
Surgical pathology showed a 4.2 cm tumor consistent with melanoma. The tissue was positive by immunohistochemistry (IHC) for S100 and SOX10. IHC negative for cytokeratin, CK7, CK20 and CDX-2 (Fig. 5). Sixteen lymph nodes were sent with the specimen and all were confirmed benign lymphoid tissue.
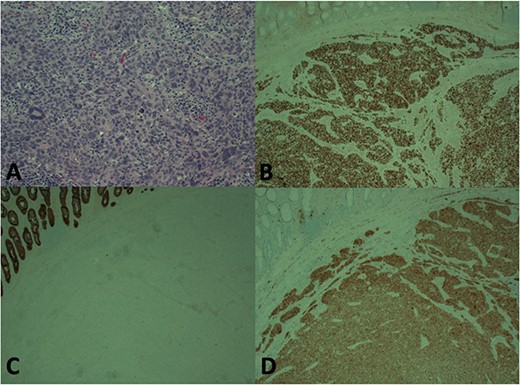
Histology showing H and E stain (A), Immunohistochemistry (IHC) for SOX-10 (B), IHC for CKC (C), IHC for S100 (D). Staining pattern consistent with melanoma
Post-discharge he has been recovering well at home. He underwent extensive skin and ophthalmologic exams that did not reveal any possible primary cutaneous melanoma. Given his dark complexion, a meticulous exam was required. He has been receiving adjuvant immunotherapy with pembrolizumab. MRI head was obtained that did not show any evidence of metastatic disease. He also will be receiving surveillance CT scans of the chest/abdomen/pelvis every 3 months for the next year.
DISCUSSION
Clinically, the most common symptoms at presentation for primary melanoma include abdominal pain and weight loss, which differs from the presenting symptoms of metastatic GI melanoma, which typically presents with bleeding, obstruction and abdominal pain [2, 5, 6, 8]. The average age of presentation is around 60 years old and without gender predilection [2].
Histologically, the diagnosis of primary colonic melanoma is usually best supported by the results of specific immunohistochemistry staining with positivity for S-100 being highly sensitive (90%) and HMB-45 with 100% specificity and 80% sensitivity [2, 6].
In a mucosal melanoma of the colon, mucosal melanoma staging is utilized. For uncommon primary mucosal melanomas, the proposed diagnostic criteria can be extrapolated to primary colonic melanoma. The strict diagnostic criteria include: (i) no previously removed pigmented skin tumors and preferably no history of skin tumor excision, (ii) no previously excised ocular tumors, (iii) the tumor must be a solitary tumor within the surgical specimen, (iv) the tumor should be morphologically compatible with a primary tumor, (v) no other melanomas in any other organs or body locations at the time of surgery and (vi) an autopsy should definitely exclude the possibility of a primary malignant melanoma elsewhere [9].
In local disease, surgical resection with wide margins is the gold standard. Surgery can also provide a palliative benefit in patients with symptomatic melanoma of the GI tract such as GI bleeding and obstruction [2]. The prognosis for primary colonic melanoma appears to be better than that of other mucosal melanomas, but mucosal melanomas tend to be more aggressive than cutaneous melanoma due to their rich lymphatic and vascular supply, difficulty in diagnosing early and potential for severe complications [2, 8]. For all primary melanomas of the GI tract, surgery improved mean survival time (MST) to about 19 months versus 8 months in nonoperative cases; however, there was not enough data on primary colonic melanoma to determine a MST [4].
Postoperatively, it is important for all patients to have a very through skin exam, rectal exam and ocular exam to rule out these possible primary sources of the melanoma. It is also prudent to have a patient see medical oncology for consideration of targeted immunotherapy [10]. PD-1 inhibitors such as pembrolizumab have shown efficacy against mucosal melanoma by increasing progression free survival with a response rate of 23%. Compared to metastatic melanoma to the colon with a 5-year survival of 33%, primary colonic melanoma seems to confer a better prognosis [7].
ACKNOWLEDGEMENTS
The authors appreciate the help of Dr Robert Pu for supplying the histology images for the paper.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
DATA AVAILABILITY
All data is available upon request.
References
- abdominal pain
- weight reduction
- cancer
- colonoscopy
- colorectal cancer
- immunologic adjuvants
- pharmaceutical adjuvants
- immunotherapy
- laparoscopy
- melanoma
- tissue membrane
- anus
- colon
- mucous membrane
- uvea
- colon cancer
- skin cancer
- computed tomography/positron emission tomography imaging
- malignant melanoma, cutaneous
- colectomy, right
- transverse colon
- abdominal bloating
- pembrolizumab
- gastroenterologists



