-
PDF
- Split View
-
Views
-
Cite
Cite
Heber B Sapan, Leo Rendy, Michael Tendean, Jimmy Panelewen, Giant gallbladder case report with vascular endothelial growth factor (VEGF) serum level and mRNA expression evaluation, Journal of Surgical Case Reports, Volume 2023, Issue 8, August 2023, rjad447, https://doi.org/10.1093/jscr/rjad447
Close - Share Icon Share
Abstract
Giant gallbladder (GGB) is a rare condition and its pathogenesis could not be explained clearly. It can result from cholelithiasis, chronic cholecystitis or neoplasm, but more rarely if created congenitally. Adequate vasculatization should support the development of this entity. A 47-year-old lady presented with a dull pain and right upper quadrant abdominal mass. A computed tomography scan showed 27 × 25 × 12 cms cystic mass expanding to the right illiac fossae, surrounded by a homogenous capsule. There were neither stones nor mass in the biliary tract and total cholecystectomy was done. Patient recovered well without signs of cholestasis 5 years postoperatively. A few cases of giant benign gall bladder have been reported in literature; however, no study has tried to investigate the mechanism of its etiology. To support the enlargement of the tissue or organ there must be some growth factors along with adequate vascularization. Vascular endothelial growth factor (VEGF) serum level and VEGF messenger ribonucleic acid (mRNA) gene expression were increased in this case. This GGB case suggests a congenital factor as its etiology. Cholecystectomy may relieve uncomfortable symptoms with good results. The incidence of GGB accompanied by increased serum VEGF levels and mRNA gene expression supports the hypothesis that VEGF plays a major role in supporting the vasculogenesis of GGBs.
INTRODUCTION
As we all know, gallbladder is a pear shaped sac, ~7–10 cm long, with an average capacity of 30 to 50 ml. In an obstructed condition, the gallbladder may dilate and contain bile up to ±300 ml [1]. There is no exact definition of a ‘giant’ gallbladder from the literature; however, the term giant gallbladder (GGB) term may be used if its weight is greater than the weight of the liver itself, which is ~1.5 kg [2]. The incidence of GGB is quite rare. Relevant studies were identified by searching PubMed with the keyword of GGB and published before May 2023. Prior to this report there were 15 similar case reports related to giant (see Table 1).
| No . | Publication . | Sex . | Age (years) . | Gallbladder size/greatest dimension (cm) . | Gallbladder Volume (liters) or weight (grams) . | Cystic duct lumen patency . | Stones . | Histopathological findings . |
|---|---|---|---|---|---|---|---|---|
| 1 | Petit (1750) [8] | F | 27 | NA | ±2 | Probably | NA | |
| 2 | Van Swieten (1754) [8] | M | 12 | NA | ±2.6 | Yes | NA | |
| 3 | Collinson [8] | NA | NA | NA | 12.5 | NA | NA | |
| 4 | Neudörfer (1911) [8] | F | 50 | NA | 5.25 | Yes | NA | |
| 5 | Kehr (1913) [9] | NA | NA | NA | 1.5 | NA | NA | |
| 6 | Grosberg (1962) [10] | F | 95 | 140 | NA | NA | Yes | Gangrenous cholecystitis |
| 7 | Maeda (1979) [11] | F | 36 | 180 | NA | NA | No | Normal gallbladder |
| 8 | Borodach et al. (2005) [2] | F | 67 | 20 × 12 | 1.5 | Yes | NA | |
| 9 | Hsu (2011) [12] | F | 87 | 16.4 × 13.6 × 7.8 | NA | NA | Yes | Adenocarcinoma |
| 10 | Panaro et al. (2012) [13] | M | 17 | 43 × 21 × 20 | 2.7 | Yes | no | Byler’s disease, normal gallbladder |
| 11 | Zong et al. (2013) [14] | F | 55 | 30 × 31 × 18 | 4 | Yes | no | Inflammatory cell infiltration |
| 12 | Kuznetzov, (2014) [2] | F | 77 | 24 × 17 | 3.3 | Yes | NA | Chronic cholecystitis |
| 13 | Yadav et al. (2017) [15] | F | 46 | 30 | NA | NA | Yes | Chronic cholecytitis with mucocele |
| 14 | Fultang et al. (2019) [8] | F | 63 | 19.5 × 5.4 × 5.6 | 73.8 g | Yes | Yes | Hemosiderinophages within wall mixed with foreign body multinucleated giant cells, no malignancy |
| 15 | Mirali (2021) [16] | F | 53 | 22 × 14 × 10 | No | Yes | chronic cholecystitis | |
| 16 | This case | F | 47 | 27 × 25 × 12 | 3.2 | Yes | chronic cholecystitis | |
| Total 16 cases | Mostly female | 52.28 (average) | Varies | ≥1.5 L | Mostly yes | varies 5 – yes 3 – no 7 – NA | varies | |
| No . | Publication . | Sex . | Age (years) . | Gallbladder size/greatest dimension (cm) . | Gallbladder Volume (liters) or weight (grams) . | Cystic duct lumen patency . | Stones . | Histopathological findings . |
|---|---|---|---|---|---|---|---|---|
| 1 | Petit (1750) [8] | F | 27 | NA | ±2 | Probably | NA | |
| 2 | Van Swieten (1754) [8] | M | 12 | NA | ±2.6 | Yes | NA | |
| 3 | Collinson [8] | NA | NA | NA | 12.5 | NA | NA | |
| 4 | Neudörfer (1911) [8] | F | 50 | NA | 5.25 | Yes | NA | |
| 5 | Kehr (1913) [9] | NA | NA | NA | 1.5 | NA | NA | |
| 6 | Grosberg (1962) [10] | F | 95 | 140 | NA | NA | Yes | Gangrenous cholecystitis |
| 7 | Maeda (1979) [11] | F | 36 | 180 | NA | NA | No | Normal gallbladder |
| 8 | Borodach et al. (2005) [2] | F | 67 | 20 × 12 | 1.5 | Yes | NA | |
| 9 | Hsu (2011) [12] | F | 87 | 16.4 × 13.6 × 7.8 | NA | NA | Yes | Adenocarcinoma |
| 10 | Panaro et al. (2012) [13] | M | 17 | 43 × 21 × 20 | 2.7 | Yes | no | Byler’s disease, normal gallbladder |
| 11 | Zong et al. (2013) [14] | F | 55 | 30 × 31 × 18 | 4 | Yes | no | Inflammatory cell infiltration |
| 12 | Kuznetzov, (2014) [2] | F | 77 | 24 × 17 | 3.3 | Yes | NA | Chronic cholecystitis |
| 13 | Yadav et al. (2017) [15] | F | 46 | 30 | NA | NA | Yes | Chronic cholecytitis with mucocele |
| 14 | Fultang et al. (2019) [8] | F | 63 | 19.5 × 5.4 × 5.6 | 73.8 g | Yes | Yes | Hemosiderinophages within wall mixed with foreign body multinucleated giant cells, no malignancy |
| 15 | Mirali (2021) [16] | F | 53 | 22 × 14 × 10 | No | Yes | chronic cholecystitis | |
| 16 | This case | F | 47 | 27 × 25 × 12 | 3.2 | Yes | chronic cholecystitis | |
| Total 16 cases | Mostly female | 52.28 (average) | Varies | ≥1.5 L | Mostly yes | varies 5 – yes 3 – no 7 – NA | varies | |
| No . | Publication . | Sex . | Age (years) . | Gallbladder size/greatest dimension (cm) . | Gallbladder Volume (liters) or weight (grams) . | Cystic duct lumen patency . | Stones . | Histopathological findings . |
|---|---|---|---|---|---|---|---|---|
| 1 | Petit (1750) [8] | F | 27 | NA | ±2 | Probably | NA | |
| 2 | Van Swieten (1754) [8] | M | 12 | NA | ±2.6 | Yes | NA | |
| 3 | Collinson [8] | NA | NA | NA | 12.5 | NA | NA | |
| 4 | Neudörfer (1911) [8] | F | 50 | NA | 5.25 | Yes | NA | |
| 5 | Kehr (1913) [9] | NA | NA | NA | 1.5 | NA | NA | |
| 6 | Grosberg (1962) [10] | F | 95 | 140 | NA | NA | Yes | Gangrenous cholecystitis |
| 7 | Maeda (1979) [11] | F | 36 | 180 | NA | NA | No | Normal gallbladder |
| 8 | Borodach et al. (2005) [2] | F | 67 | 20 × 12 | 1.5 | Yes | NA | |
| 9 | Hsu (2011) [12] | F | 87 | 16.4 × 13.6 × 7.8 | NA | NA | Yes | Adenocarcinoma |
| 10 | Panaro et al. (2012) [13] | M | 17 | 43 × 21 × 20 | 2.7 | Yes | no | Byler’s disease, normal gallbladder |
| 11 | Zong et al. (2013) [14] | F | 55 | 30 × 31 × 18 | 4 | Yes | no | Inflammatory cell infiltration |
| 12 | Kuznetzov, (2014) [2] | F | 77 | 24 × 17 | 3.3 | Yes | NA | Chronic cholecystitis |
| 13 | Yadav et al. (2017) [15] | F | 46 | 30 | NA | NA | Yes | Chronic cholecytitis with mucocele |
| 14 | Fultang et al. (2019) [8] | F | 63 | 19.5 × 5.4 × 5.6 | 73.8 g | Yes | Yes | Hemosiderinophages within wall mixed with foreign body multinucleated giant cells, no malignancy |
| 15 | Mirali (2021) [16] | F | 53 | 22 × 14 × 10 | No | Yes | chronic cholecystitis | |
| 16 | This case | F | 47 | 27 × 25 × 12 | 3.2 | Yes | chronic cholecystitis | |
| Total 16 cases | Mostly female | 52.28 (average) | Varies | ≥1.5 L | Mostly yes | varies 5 – yes 3 – no 7 – NA | varies | |
| No . | Publication . | Sex . | Age (years) . | Gallbladder size/greatest dimension (cm) . | Gallbladder Volume (liters) or weight (grams) . | Cystic duct lumen patency . | Stones . | Histopathological findings . |
|---|---|---|---|---|---|---|---|---|
| 1 | Petit (1750) [8] | F | 27 | NA | ±2 | Probably | NA | |
| 2 | Van Swieten (1754) [8] | M | 12 | NA | ±2.6 | Yes | NA | |
| 3 | Collinson [8] | NA | NA | NA | 12.5 | NA | NA | |
| 4 | Neudörfer (1911) [8] | F | 50 | NA | 5.25 | Yes | NA | |
| 5 | Kehr (1913) [9] | NA | NA | NA | 1.5 | NA | NA | |
| 6 | Grosberg (1962) [10] | F | 95 | 140 | NA | NA | Yes | Gangrenous cholecystitis |
| 7 | Maeda (1979) [11] | F | 36 | 180 | NA | NA | No | Normal gallbladder |
| 8 | Borodach et al. (2005) [2] | F | 67 | 20 × 12 | 1.5 | Yes | NA | |
| 9 | Hsu (2011) [12] | F | 87 | 16.4 × 13.6 × 7.8 | NA | NA | Yes | Adenocarcinoma |
| 10 | Panaro et al. (2012) [13] | M | 17 | 43 × 21 × 20 | 2.7 | Yes | no | Byler’s disease, normal gallbladder |
| 11 | Zong et al. (2013) [14] | F | 55 | 30 × 31 × 18 | 4 | Yes | no | Inflammatory cell infiltration |
| 12 | Kuznetzov, (2014) [2] | F | 77 | 24 × 17 | 3.3 | Yes | NA | Chronic cholecystitis |
| 13 | Yadav et al. (2017) [15] | F | 46 | 30 | NA | NA | Yes | Chronic cholecytitis with mucocele |
| 14 | Fultang et al. (2019) [8] | F | 63 | 19.5 × 5.4 × 5.6 | 73.8 g | Yes | Yes | Hemosiderinophages within wall mixed with foreign body multinucleated giant cells, no malignancy |
| 15 | Mirali (2021) [16] | F | 53 | 22 × 14 × 10 | No | Yes | chronic cholecystitis | |
| 16 | This case | F | 47 | 27 × 25 × 12 | 3.2 | Yes | chronic cholecystitis | |
| Total 16 cases | Mostly female | 52.28 (average) | Varies | ≥1.5 L | Mostly yes | varies 5 – yes 3 – no 7 – NA | varies | |
The pathogenesis of a GGB could not be explained clearly. Courvoisier (1890) stated that an enlarging gallbladder is more frequently caused by a biliary obstructive tumor than gallstones [3]. A gallstone does not result in an enlarged gallbladder, as it is formed over an extend period of time, resulting in a shrunken, fibrotic, and scarred gallbladder that does not allow for extended enlargement [3]. However, this process may not always be compatible with how GGB is formed. A GGB may be congenitally or secondarily formed due to neoplasm growth or gallstones, which disturb the drainage of the bile and, in turn, cause the dilatation of the gallbladder. Neoplasm and bile obstruction may extend the size of gallbladder to an unlimited size. Aside from the factors above when there are no neoplasm or cholestasis conditions, we postulate that there must be good vascularization to support the hyperplasia of the gallbladder [2]. Experimental studies showed that vascular endothelial growth factor (VEGF) is essential for muscle growth and good angiogenesis in embryonic, postnatal development, and even adult life is very much supported by the main role of VEGF [4–6].
This case report presents a GGB case in a 47-year-old woman, followed by an investigation of the VEGF serum level and messenger ribonucleic acid (mRNA) gene expression of the patient together with her family in order to predict the inheritance factor of the VEGF mRNA gene expression in the incidence of GGB. The case report adheres to SCARE criteria [7].
CASE PRESENTATION
A 47-year-old female presented with a mass at the right upper quadrant of the abdomen accompanied by dull pain since 5 years prior to admission, and in the last 2 weeks, that abdominal mass felt progressively enlarged by the patient. There were no signs and symptoms of bile obstruction or bowel problems. There was no history of fever or weight loss accompanying the chief complaint.
The abdominal wall appeared asymmetrical due to the right upper abdominal mass. Abdominal palpation found a mass with a size of 27 × 25 × 12 cm, a mobile, cystic, smooth surface and no tenderness. This cystic mass extends from the right hypochondriac to the right illiac region of the abdomen. Laboratory test results were unremarkable except for mild leukocytosis (13 500/mm [3]). Ultrasound and computed tomography (CT)-scan showed a cystic mass suggesting a very large gallbladder, expanding from the right hypochondriac to the right illiac fossae of the peritoneal cavity, surrounded by a homogenous capsule (see Fig. 1). The differential impression of this imaging was a mesenterial cyst. After that, the patient was scheduled for elective surgery.
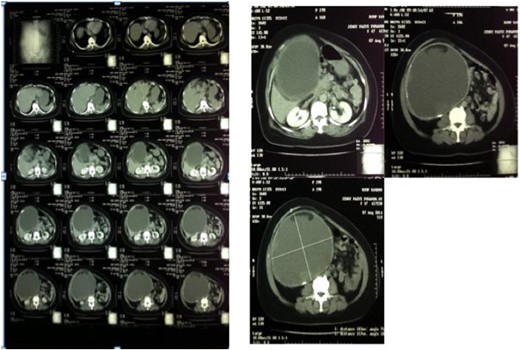
Abdominal CT-scan showed homogenous cystic mass surrounded with a regular wall (left). A cystic mass below the liver expanded to the right lower quadrant of the abdomen (right).
During laparotomy, we discovered a balloon-shaped cystic mass surrounded by omentum and adherent to surrounding tissues (see Fig. 2). About 3.2 L of golden brown liquid within the cyst were aspirated. Further identification of the cyst revealed this lesion as GGB instead of mesenteric cyst, as suggested by the imaging result. During the evaluation of the hepatobiliary tract, there were neither stones nor mass in the biliary tract. This cystic mass was further separated from liver (see Fig. 2C) and finally removed by total cholecystectomy. The bile was aspirated and sent for culture, which was sterile.
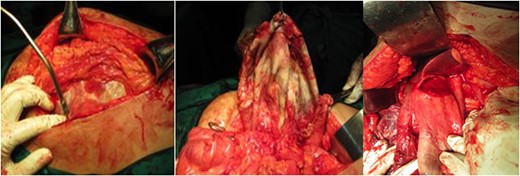
Intraoperative findings showed a balloon-shaped cystic mass surrounded by omentum and adherent to surrounding tissues. (A) A cystic mass was surrounded by omentum, (B) The cystic mass had been dissected from the mesentery and surrounding tissue and found out to be a GGB. (C) The cystic mass appears on the inferior surface of the liver, where the gallbladder’s anatomical position should be.
There was no problem with the postoperative period, and the patient was discharged 5 days after surgery. Up until the 2-year follow-up, the patient felt relieved and asymptomatic without signs of cholestasis.
VEGF investigation
We collect the family members of the GGB patient with the family pedigree (see Fig. 3) to take the sample of VEGF serum level and mRNA gene expression from each family member.
![Pedigree of the patient’s family in sampling. [1] Mrs. YP, 48 years old (Patient); [2] Mr. HL, 49 years old (husband); [3] Ms. ML, 17 years old (1st children); [4] Mr. ML, 5 years old (2nd children); [5] Mr. YP, 56 years old (brother); [6] Mrs. DP, 61 years old (sister); [7] Mr. SK, 81 years old (uncle from mother side); [8] Mrs. MP, 62 years old (aunt from father side); [9] Mrs. BP, 66 years old (aunt from father side); [10] Mr. CP, 76 years old (uncle from father side).](https://oupdevcdn.silverchair-staging.com/oup/backfile/Content_public/Journal/jscr/2023/8/10.1093_jscr_rjad447/1/m_rjad447f3.jpeg?Expires=1773834954&Signature=WpFvczftxfjBhcdQ3olYCJfUMa3BxeTuxlnklBVr2z5Zpo~LxgZ82PS03PrDQbZra~MI5ibXFxuc6jDTafbS34iRzhgGwC0SqDJFVtWYhFMnEVSl-bHVOndSGIsZhbXaaJNghbuJSv7HT99D-m~eHny3E~KpIj7c2fOznIw4nw7FEO7K2owTWQAY5evDnYk~MsNssWvx6meP98~lgw33nC6zfjeGoTGYfTVb0glHRVK1uBLwSMOKqgt-C497M0Gh04tSfO1lRtE-l7QIWbeh7IkfuCRjsGH~bQ2wCjye0hYNjkcplZ1zaLe-AtMM4Ggxsnwk~sDWPmaqzxttvhgQGQ__&Key-Pair-Id=APKAIYYTVHKX7JZB5EAA)
Pedigree of the patient’s family in sampling. [1] Mrs. YP, 48 years old (Patient); [2] Mr. HL, 49 years old (husband); [3] Ms. ML, 17 years old (1st children); [4] Mr. ML, 5 years old (2nd children); [5] Mr. YP, 56 years old (brother); [6] Mrs. DP, 61 years old (sister); [7] Mr. SK, 81 years old (uncle from mother side); [8] Mrs. MP, 62 years old (aunt from father side); [9] Mrs. BP, 66 years old (aunt from father side); [10] Mr. CP, 76 years old (uncle from father side).
VEGF serum level
The result of VEGF serum level from each family member showed that patient and patient’s uncle had significantly higher VEGF serum level compared with the rest of family (see Fig. 4).
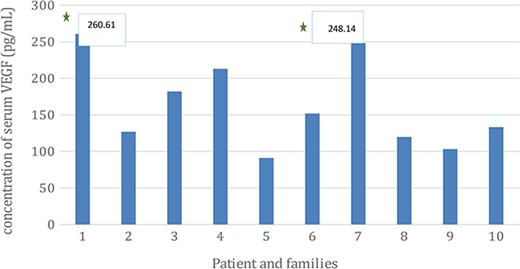
The concentration of VEGF serum level of the GGB patient and among family members. The level of VEGF serum level were (1) 260.61 pg/mL; (2) 126.89 pg/mL; (3) 182.06 pg/mL; (4) 212.92 pg/mL; (5) 91.05 pg/mL; (6) 151.83 pg/mL; (7) 248.14 pg/mL; (8) 119.73 pg/mL; (9) 103.21 pg/mL; (10) 133.13 pg/mL.
Expression of VEGF mRNA gene
The expression of VEGF gene mRNA among the family member had similar pattern with the serum level result which showed that patient and patient’s uncle once again significantly had higher level of VEGR gene mRNA expression (see Fig. 5).
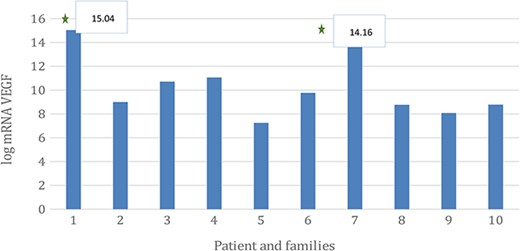
The expression level of VEGF gene mRNA in the GGB patient and among family members. The VEGF serum level were (1) 15.04; (2) 8.99; (3) 10.71; (4) 11.06; (5) 7.25; (6) 9.77; (7) 14.16; (8) 8.76; (9) 8.07; (10) 8.78.
Histopathology examination of giant gallbladder tissue
Histopathologic examination showed tall columnar epithelia with cholesterol and fat globules, and the intra-abdominal cyst resembled the histopathologic feature of a vesical fellea with infiltration of lymphocytes and macrophages, brown pigments and necrotic tissue, which indicated signs of chronic inflammation of the gallbladder (see Fig. 6).
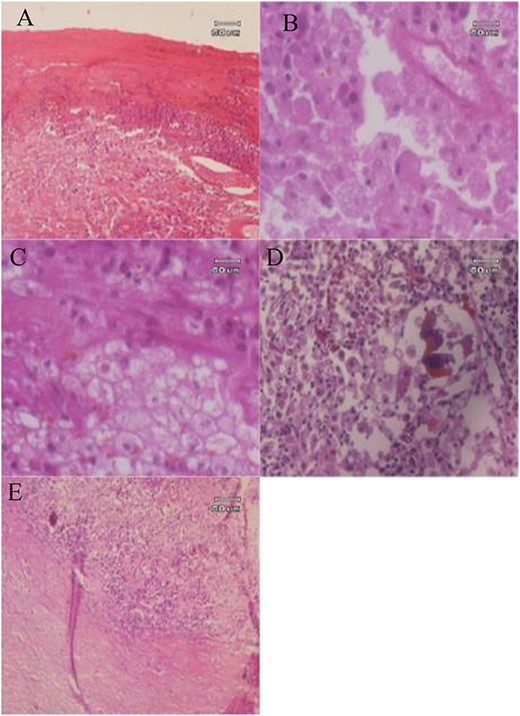
Histopathology examination of GGB: (A) The cyst wall consists of fibrous tissue infiltrated by inflammatory cells; (B) macrophage foamy cells; (C, D) foamy macrophages and brown pigments; (E) Fibrous tissue with infiltration of mononuclear inflammatory cells.
DISCUSSION
The GGB is a unique clinical entity, and its incidence is very rare [2]. Earlier case reports discussed that the pathologies for GGBs were chronic inflammation (cholecystitis), congenital, diabetes, visceromegaly due to acromegaly, inappropriate accumulation of mucus (mucocele), malignancy (adenocarcinoma), cholelithiasis and cholestatic liver disease (Byler’s disease) [12].
Reports of GGB are rare; however extremely large gallbladders without obstructive factors such as biliary tumors and gallstones is much rarer [8–16]. Intermittent bile obstruction may cause gallbladder valve mechanism to trap bile inside. This situation causes the gallbladder to dilate progressively, and with exclusive conditions such as minimal bacterial contamination and adequate vascularization, the gallbladder may dilate to a significant size. With the expansion of volume, the contractile function of the muscular wall of the gallbladder may be distressed, resulting in a GGB.
Other etiologies that may impair bile flow include impairment of innervation. In younger patients, congenital anomaly such as hypoaganglionosis of cystic duct could result in enlargement of the gallbladder [1].
The case report we present here is unique due to the unusual large size of the gallbladder without obstructive factors. In this case, the GGB was not caused by a tumor or gallstones, and there were also no signs of bile obstruction in intrahepatic and extrahepatic bile tract. The fact that the bile content was not white bile indicates the patency of the cystic duct. A significant infection was also not the case. Therefore, we postulate that this GGB case is due to a congenital factor.
To support the enlargement or growth of the tissue or organ there must be some growth factors which has some role. We want to investigate the relation of the VEGF gene expression and GGB incidence. As explained above, VEGF plays a major role in vasculogenesis and angiogenesis. In the condition of so-called GGB, one of the supporting factors that can support a gallbladder enlargement to an unlimited size must be adequate vascularization to its tissue, despite the underlying etiology of the GGB itself.
The results from the examination of VEGF serum level showed increases in the patient (sample 1) (260.6133015) and patient’s uncle from mother’s side (sample 7) (284.1454419), and in line with the serum level, mRNA expression of VEGF gene was also increased (see Fig. 4). The increase in VEGF serum levels and VEGF gene mRNA expression was consistent with the theory of adequate vasculogenesis and angiogenesis to support the higher congenital growth factors as the etiology of GGBs.
From the examination of the patient’s families, it is hard to determine that the increased VEGF mRNA gene expression is an inherited trait from both of the patient’s parents since the parents had passed away and hence examination could not be done. Another thing that could be concluded from the results is that the increased VEGF mRNA gene expression might be inherited from the mother side of the patient; this can be seen from the result of the brother of patient’s mother (sample 7, see Figs 4 and 5).
Later studies are still needed to confirm the relationship between VEGF concentration and GGB incidence, accompanied by more samples from different GGB cases.
CONCLUSION
A GGB is a rare pathologic entity and can be found without an obstructive condition, as in this case, suggesting a congenital factor as its etiology. Cholecystectomy to relieve uncomfortable symptoms due to an enlarged abdominal mass may be considered with good results.
The incidence of GGB accompanied by increased serum VEGF levels and mRNA gene expression supports the hypothesis that VEGF plays a major role in supporting the vasculogenesis of GGBs.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
DATA AVAILABILITY
Data can be obtained by emailing the corresponding author.
ETHICAL APPROVAL
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.



