-
PDF
- Split View
-
Views
-
Cite
Cite
Ines Lebbe, Eveline De Cuypere, Nele Claes, Anne Loccufier, Christophe Ghysel, Philippe Van Trappen, Primary leiomyosarcoma of the uterine cervix: an unusual case and critical appraisal, Journal of Surgical Case Reports, Volume 2023, Issue 8, August 2023, rjad439, https://doi.org/10.1093/jscr/rjad439
Close - Share Icon Share
Abstract
Leiomyosarcomas of the uterine cervix are rare, mostly occurring in perimenopausal women. Diagnosis is based on pathology and immunohistochemistry. Surgery with a total abdominal hysterectomy and bilateral salpingo-oophorectomy remains the standard. A female patient in her 60s presented with heavy postmenopausal bleeding. Vaginal ultrasound scan and magnetic resonance imaging showed a large strongly vascularized cervical mass with features suspicious of sarcomatous degeneration. Positron Emission Tomography-Computed Tomography (PET-CT) did not reveal any evidence of metastases nor lymphadenopathy, but presence of right hydronephrosis. An abdominal hysterectomy with bilateral salpingo-oophorectomy, and end-to-end anastomosis of the right ureter, was performed. Pathology showed an International Federation of Gynecology and Obstetrics (FIGO)-stage 1B leiomyosarcoma of the uterine cervix. No adjuvant treatment was given. Adjuvant radiotherapy reduces the risk of recurrence but no survival impact. The benefit of adjuvant chemotherapy is questionable given the lack of randomized trials. Multidisciplinary research concerning molecular alterations of the disease is required to determine optimal management strategies with potential novel molecular therapies.
INTRODUCTION
Sarcomas of the female genital tract account for ~3% of all gynecological malignancies, with uterine sarcomas being the most common subtype in ~80% of the cases [1]. Cervical leiomyosarcomas constitute <1% of all cervical malignancies and they mostly occur in perimenopausal women with an average age at diagnosis of 46 years [2]. Of all cervical sarcomas, carcinosarcomas (malignant mixed Müllerian tumors) are the most common subtype, with cervical leiomyosarcoma being the second most common, followed by adenosarcoma, rhabdomyosarcoma and endometrial stromal sarcoma [3]. Clinical symptoms typically include abnormal vaginal blood loss, abdominal distension, a cervical mass and pelvic pain. Preoperative imaging characteristics with ultrasound scan and/or magnetic resonance imaging (MRI) have been investigated and can raise suspicion of a sarcoma [4]. With ultrasound scan, uterine leiomyosarcomas are often larger in size, solitary and with increased central/peripheral vascularity when compared with benign uterine masses [5]. The final diagnosis is based on pathology and immunohistochemical analysis [6].
The standard surgical care is a total abdominal hysterectomy and bilateral salpingo-oophorectomy. Adjuvant radiotherapy in uterine sarcomas has shown in a few studies to reduce the risk for local recurrence, but no benefit on overall survival (OS) [7]. The benefit of adjuvant chemotherapy is questionable given the controversial findings in studies in uterine sarcomas [3].
Several poor prognostic factors have been identified including tumor size, stage, grade, mitotic count, age and postmenopausal status [8].
Here we describe a case of a female patient in her 60s who was diagnosed with a bulky primary cervical leiomyosarcoma, treated by abdominal hysterectomy and bilateral salpingo-oophorectomy.
CASE REPORT
A female patient in her 60s presented at the emergency department with heavy postmenopausal blood loss. Her medical history includes hypercholesterolemia and two vaginal deliveries (G2P2A0M0). She was allergic to penicillin. Her mother was recently diagnosed with breast cancer.
Clinical vaginal examination revealed a circumferentially distended uterine cervix. Vaginal ultrasound scan and magnetic resonance imaging (MRI) showed a strongly vascularized mass that completely involved the whole cervix. The mass measured 118 × 117 × 124 mm and showed irregular echogenicity with signs of diffuse necrosis centrally, suspicious for sarcomatous degeneration (Fig. 1). Positron emission tomography-computed tomography (PET-CT) showed a heterogenous and irregular hypermetabolic uterus, suspected for malignancy (Fig. 2). There were no signs of distant metastases nor lymphadenopathy. PET-CT also showed a distended ureter with hydroureteronephrosis on the right side, suspicious of compression/obstruction of the right ureter (Figs 2–4).
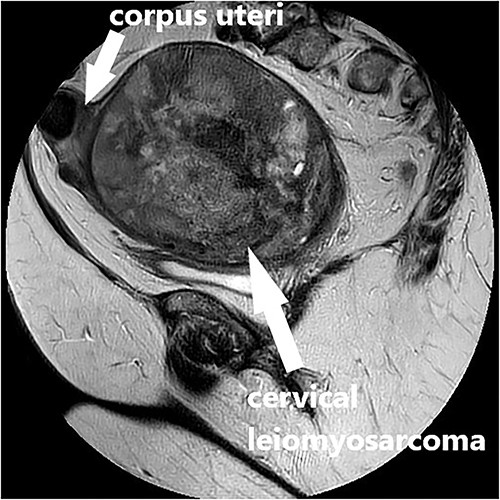
MRI scan, T2-weighted sagittal image of the large vascularized cervical mass in the pelvis with heterogenous and degenerative changes, and corpus uteri on top.
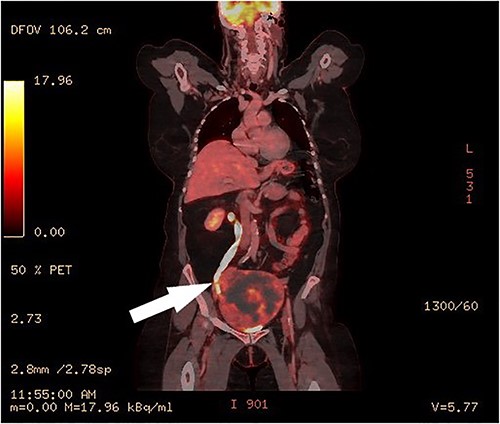
PET-CT, coronal image of a dilated right ureter with stenosis at the pelvic mass.
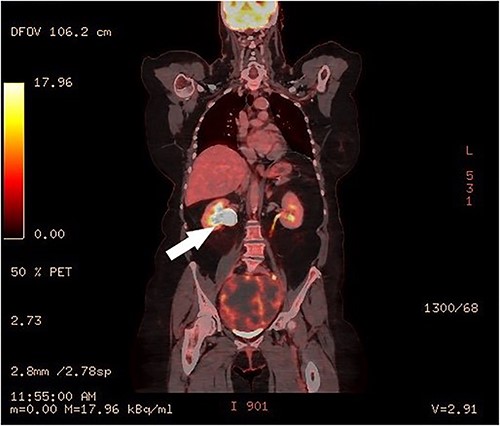
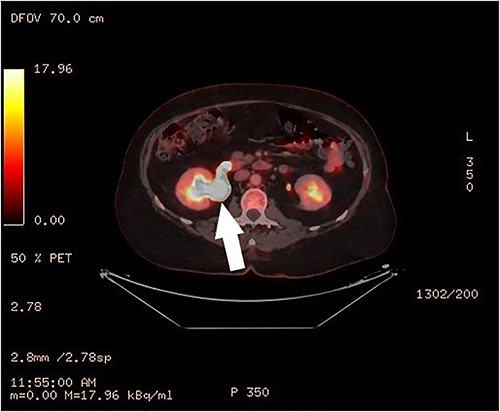
Surgery included a total abdominal hysterectomy (via median laparotomy) and bilateral salpingo-oophorectomy, and end-to-end anastomosis of the right ureter. Frozen section procedure of the uterus confirmed the diagnosis of a cervical leiomyosarcoma. The right ureter was completely stuck and adherent to the cervical mass and appeared to be obliterated. Partial resection of 2 cm of obliterated ureter was performed, with subsequent end-to-end anastomosis and double-J stent of the right ureter by the urologist. The recovery went uneventful and the patient was discharged on Day 7 postoperatively. The urinary catheter was removed after 14 days. The double J stent was removed by cystoscopy by the urologists after 6 weeks.
Macroscopic pathology report showed a uterine weight of 1397 g. The uterine corpus measured 4 × 4.5 × 3 cm, with a mass in the cervix of 15 × 13 × 12 cm. Microscopic findings included a spindle-cell neoplasm with high cellularity, cytonuclear atypia, high mitotic activity and necrosis, suspected for leiomyosarcoma. There were no signs of lymphovascular nor perineural invasion. Immunohistochemical examination was positive for smooth muscle actin (SMA), Desmin and Caldesmon. Intravascular positivity of CD34, CD34 was negative in spindle cells. PHH3 confirms the high mitotic activity. P53 was positive in the spindle cells; ATRX was negative. She was staged as an International Federation of Gynecology and Obstetrics-stage 1B or pT1b leiomyosarcoma of the uterine cervix.
Multidisciplinary oncologic advice was no adjuvant therapy. Postoperative follow-ups on a 4-monthly basis were uneventful.
DISCUSSION
Here we describe in a postmenopausal woman in her 60s a large uterine cervical leiomyosarcoma extending to both pelvic side walls and obstructing the right ureter. She underwent a total abdominal hysterectomy, bilateral salpingo-oophorectomy and end-to-end anastomosis of the right ureter. In our case, the patient had a leiomyosarcoma of 15 cm.
The 5-year OS of cervical sarcomas depends on the subgroup. In a study of 1370 women with cervical sarcoma of the National Cancer Database, the 5-year OS is 89.2% for cervical adenosarcoma, 66.2% for rhabdomyosarcoma, 55.6% for leiomyosarcoma, 45.8% for endometrial stromal sarcoma, 31.6% for carcinosarcomas and 21.2% for sarcoma not otherwise specified [3].
Imaging with ultrasound scan and/or MRI can raise the suspicion preoperatively of a uterine/cervical sarcoma on the basis of certain features [1]. They tend to be larger and solitary compared with benign uterine/cervical masses, with heterogeneous characteristics including necrosis and increased central/peripheral vascularity. In addition to imaging characteristics, with in particular degenerative changes, the value of raised serum lactated dehydrogenase has shown to be a significant predictor for uterine malignancy in a retrospective study [9].
The final diagnosis is based on pathology and immunohistochemical analysis. Leiomyosarcomas are malignant tumors composed of cells showing smooth muscle features [6]. Immunohistochemically, key markers used for differentiating uterine sarcoma from other tumors are SMA, desmin and vimentin.
Multiple surgical techniques have been described, such as extrafascial hysterectomy, modified radical hysterectomy and radical hysterectomy with or without retroperitoneal lymph node dissection [9]. However, the type of hysterectomy does not have an effect on the oncological outcome, as long as there are free surgical margins.
Surgery remains the standard of care for both early and advanced stage uterine sarcoma, with a total abdominal hysterectomy and bilateral salpingo-oophorectomy, with free surgical margins and without morcellation or fragmentation of the tumor [10].
Adjuvant radiotherapy gives a risk reduction in pelvic relapse in high-risk patients; however, no statistical significant benefit in OS [7].
The value of adjuvant chemotherapy is questionable. Given the risk for hematogenous spread, chemotherapy can be used in advanced/metastatic, unresectable or recurrent leiomyosarcomas [11, 12].
Molecular analyses of uterine leiomyosarcomas have revealed common genetic alterations, mutations or deletions, in several tumor suppressor genes including TP53, PTEN and RB1 [13]. Furthermore, recurrent alterations were found in telomere maintenance genes such as ATRX and homologous recombination DNA repair genes. Three molecular subtypes have been described for leiomyosarcoma, with a subgroup of uterine leiomyosarcomas behaving as an independent molecular subtype with worse survival (subtype III). An overview of recent Phase II and III trials in advanced (uterine) leiomyosarcoma, with chemotherapy, molecular targeted therapies or immunotherapy, has been published by Lacuna et al [13].
CONCLUSION
Cervical leiomyosarcomas account for <1% of all cervical malignancies. Since the rarity of the disease, no consensus has been established on the optimal surgical and adjuvant treatment. Multidisciplinary research, concerning the biological behavior and associated molecular alterations of the disease, is required to determine the optimal management strategy with potential novel molecular therapies.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
CONSENT DECLARATION
Informed consent from the patient and local ethical approval (IRB-3217) was obtained for publication of this case report and accompanying figures.
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.



