-
PDF
- Split View
-
Views
-
Cite
Cite
Takashi Miyata, Hisashi Nishiki, Yuki Shinden, Shota Motoyama, Yuta Sannomiya, Hozumi Tamezawa, Taigo Nagayama, Akifumi Hashimoto, Daisuke Kaida, Tomoharu Miyashita, Hideto Fujita, Nobuhiko Ueda, Hiroyuki Takamura, A case of anaplastic carcinoma of the pancreas with intrasplenic huge mass formation, Journal of Surgical Case Reports, Volume 2023, Issue 6, June 2023, rjad349, https://doi.org/10.1093/jscr/rjad349
Close - Share Icon Share
Abstract
Anaplastic carcinoma of the pancreas (ACP) is an aggressive pancreatic tumor that grows rapidly, and its clinical characteristics are poorly defined because of its rarity. Thus, preoperative diagnosis is difficult and most definitive diagnoses are generally made by surgery, highlighting the importance of collecting more cases of ACP. We report a case of a 79-year-old woman with ACP that was difficult to diagnose preoperatively. Abdominal enhanced computed tomography revealed a large and expansive tumor in the spleen containing multilocular cystic and solid components. The first preoperative diagnosis was splenic angiosarcoma, and the tumor could be resected by distal pancreatectomy, total gastrectomy and partial transverse colectomy. ACP was first diagnosed based on postoperative histopathological findings. ACP that spreads to the spleen and forms an intrasplenic mass is rare. However, ACP should be included in the differential diagnosis of such patients, and further research of ACP is essential for a favorable prognosis.
INTRODUCTION
Anaplastic carcinoma of the pancreas (ACP) is a rare aggressive pancreatic tumor accounting for only 0.08% of all pancreatic cancers [1], 0.5% of resected pancreatic cancers [2] and 1.4% of resected pancreatic ductal adenocarcinomas [3]. ACP is classified as a variant of ductal adenocarcinoma, generally considered to grow rapidly, and with a large tumor diameter. However, its clinical characteristics remain unknown because of its rarity and aggressiveness; therefore, preoperative diagnosis is difficult and most definitive diagnoses are made by surgery [4, 5]. Here, we report a case of ACP that was difficult to diagnose with marked infiltration of the spleen that was preoperatively diagnosed as a huge splenic tumor.
CASE REPORT
A 79-year-old woman was referred to our hospital because of nausea for about 3 months. Her medical and family histories were unremarkable. On admission, her vital parameters were as follows: temperature, 36.6°C; blood pressure, 120/84 mmHg; heart rate, 72 beats per minute (regular); respiratory rate, 15 breaths per minute and oxygen saturation, 99% on room air. Physical examination revealed a soft mass was palpable in the left upper quadrant. Laboratory investigation revealed slight anemia (hemoglobin: 10.3 g/dL), but other results, including the serum tumor markers carcinoembryonic antigen, cancer antigen 19-9 and cancer antigen 125, were within normal ranges. Abdominal computed tomography (CT) revealed a large tumor with multilocular cystic and solid components, and only a small amount of normal splenic parenchyma remained. And the tumor extensively compressed the stomach ventrally. The boundary between the pancreatic tail and the hilum of the spleen was somewhat unclear, but no obvious mass lesion was observed in the pancreas (Fig. 1A and B). On magnetic resonance imaging, the solid part included an uneven high signal on T1-weighted images and a low signal on T2-weighted images, suggesting internal bleeding (Fig. 2A and B). On a positron emission tomography scan, 35.9 points of 18F-fluorodeoxyglucose accumulation of standardized uptake value showed in the only solid part of the tumor (Fig. 3). Although a definitive preoperative diagnosis could not be made, angiosarcoma originating in the spleen was suspected first, and laparotomy was performed.
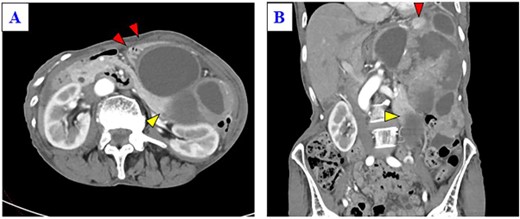
(A) Enhanced CT shows a huge mass lesion with a mixture of cystic and solid parts, occupying the left upper quadrant and flank, and the tumor expansively compressed the stomach ventrally (red arrows). (B) Only a small portion of the normal spleen parenchyma remains (red arrow). The tail of the pancreas is partially in contact with the tumor (yellow arrow), but the border of the pancreas is relatively smooth.
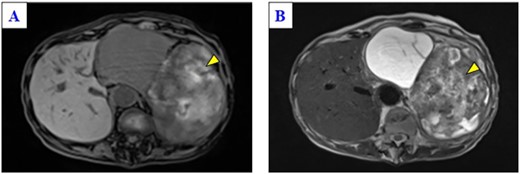
Magnetic resonance imaging of the solid part of the tumor shows a heterogeneous hyperintense area in a T1-weighted image (A) and a hypointensity area in a T2-weighted image (B) (yellow arrows).
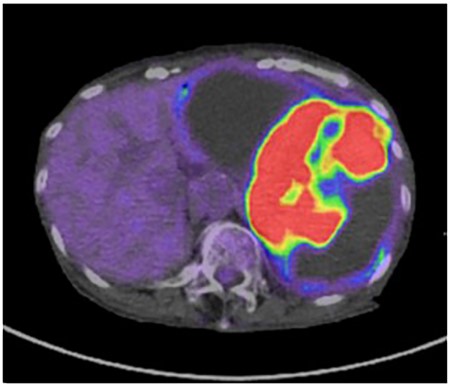
The solid part of the tumor has a higher uptake of 18F-fluorodeoxyglucose (standardized uptake value of 35.9) on positron emission tomography, and no obvious evidence of distant metastasis is present.
The tumor was located on the posterior wall of the body of the stomach and had invaded part of the transverse colon (Fig. 4A and B). After resection of the duodenum and partial resection of the transverse colon, the pancreas was divided at the left side of the portal vein. Then, the pancreatic body was mobilized by the resection of the left anterior renal fascia, and the esophagus was resected and removed the specimen (Fig. 4C). Finally, gastrointestinal reconstruction and cholecystectomy were performed. The duration of surgery was 300 min. The gross appearance was a soft brownish area with clots and mucus, a homogeneous solid gray matter area, and a cystic area, measuring 24 × 15 cm, and most of the tumor was within the spleen (Fig. 5). Microscopically, the adenocarcinoma was in contact with pancreatic tissue, with tubular and papillary growth, and pleomorphic cells were dominant, and two of the 15 lymph nodes examined were tumor-positive (Fig. 6). Immunohistochemistry was positive for CK7, CK19, vimentin, cytokeratin AE1/AE3 and negative for CK20. Therefore, a diagnosis of pleomorphic anaplastic carcinoma of the pancreas of clinical stage IIB (T3N1M0) was made according to the International Union Against Cancer tumor, nodes, and metastasis staging system. The patient was discharged 15 days after surgery, and adjuvant chemotherapy, gemcitabine with nab-Paclitaxel (GnP), was introduced after discharge. However, liver metastases appeared 5 months after her operation.
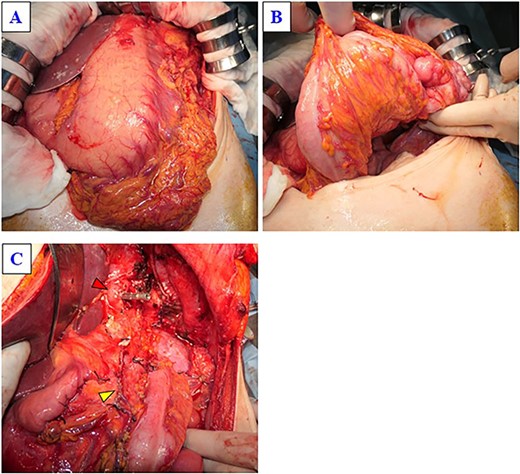
(A) The tumor is located in the posterior wall of the stomach, extending from the body to the gastric cardia, with a diameter of ~25 cm. (B) The tumor has invaded part of the transverse colon. (C) This image was obtained after the specimen was removed. The yellow arrow indicates the stump of the pancreas, and the red arrow indicates the stump of the esophagus.
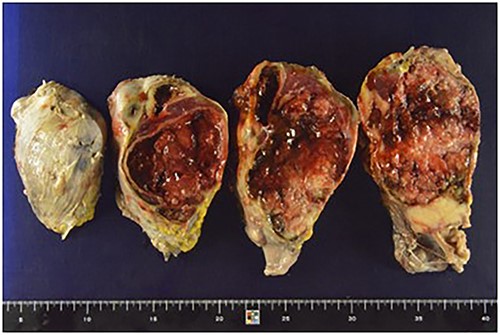
The resected specimen, 24 × 15 cm, was a solid yellowish tumor with a fibrous capsule and central necrotic change, growing inside the spleen, and also partly touching the pancreatic tail.
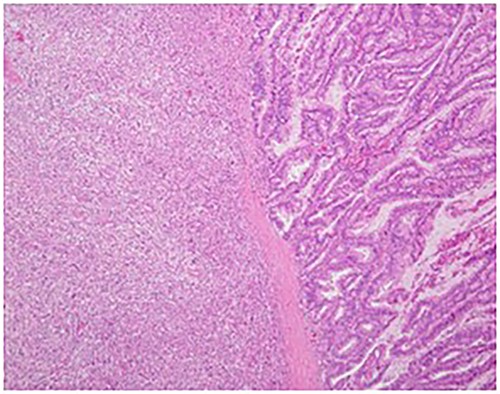
Hematoxylin–eosin staining showed spindle cells and multinuclear giant cells, which are characteristic of anaplastic carcinoma of the pancreas (original magnification: ×30).
DISCUSSION
ACP was first reported as a pleomorphic carcinoma by Sommers and Meissner in 1954, introduced as a rare pancreatic malignant tumor that showed hemorrhagic necrosis and sarcomatous growth and consisted of giant cells and pleomorphic cells [6]. According to the guidelines of the World Health Organization in 2010, ACP was classified as a pancreatic undifferentiated (anaplastic) carcinoma [7]. However, even then, because of its rarity and aggressiveness with a poor prognosis, its clinical characteristics and diagnostic methods have been poorly documented.
ACP has no specific symptoms, and physical examination does not often reveal abnormalities even if the disease is significantly advanced. Blood examination are often inconclusive [8]. Although it is difficult to make a definitive diagnosis of ACP by examination, ACP tends to induce cystic changes because of central necrosis, bleeding of degeneration related to the aggressiveness and rapid growth of ACP, unlike other types of invasive pancreatic ductal carcinomas [9, 10]. Therefore, it is important to differentiate ACP from pancreatic tumor disease with cystic lesions, such as neuroendocrine tumors and solid and pseudopapillary tumors [11, 12].
In the present case, it was considered that the APC developed in the tail of the pancreas, expanded outside the pancreas toward the spleen and formed a mass with cystic lesions within the spleen. Indeed, the imaging findings of the tumor indicated a solid tumor with cystic changes, which was consistent with APC. However, preoperative diagnosis was difficult, and surgery was performed with primary splenic angiosarcoma as the first differential diagnosis. To the best of our knowledge, this is the first report of a patient with ACP with intrasplenic mass formation.
Treatment strategies for ACP have not been established. However, it is characterized by rapid expansion and invasive growth, and is often diagnosed as unresectable; therefore, patients with ACP should be offered pancreatic resection whenever possible [13]. Unfortunately, surgery alone results in a poor prognosis, especially in ACP, which usually has a more severe prognosis than pancreatic ductal adenocarcinoma [9, 13]. Evidence of the efficacy of chemotherapy to improve the prognosis of ACP is still needed, although some case reports have demonstrated a reduction in the tumor mass and prolongation of survival after treatment with 5-fluorouracil, gemcitabine and paclitaxel [14, 15].
In conclusion, ACP that spreads and forms an intrasplenically mass is extremely rare, and therefore, it is difficult to make a definitive diagnosis prior to surgery. However, ACP should be included in the differential diagnosis of patients with its finding of intrasplenic huge mass formation, and treatment and further research of ACP are essential for a favorable prognosis.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
FUNDING
No authors have direct or indirect commercial and financial interests associated with the publication of this article.
CONSENT
The patient discussed in this case report provided their informed consent for the publication of their information.
References
- computed tomography
- gastrectomy
- cysts
- differential diagnosis
- pancreatic neoplasms
- preoperative care
- surgical procedures, operative
- abdomen
- diagnosis
- neoplasms
- pancreas
- spleen
- surgery specialty
- undifferentiated carcinoma
- colectomy, transverse
- pancreatectomy, distal
- angiosarcoma of spleen
- histopathology tests



