-
PDF
- Split View
-
Views
-
Cite
Cite
Nadia Hui Shan Sim, Sabrina Ngaserin, Jia Jun Feng, Yee Onn Kok, Hui Wen Chua, Jorge Gaviria-Pinzon, Khong Yik Chew, Allen Wei-Jiat Wong, Reconstruction of a massive chest wall defect using a free anterolateral–lower medial thigh flaps: a case report, Journal of Surgical Case Reports, Volume 2023, Issue 5, May 2023, rjad264, https://doi.org/10.1093/jscr/rjad264
Close - Share Icon Share
Abstract
Critical defects of the chest wall require robust soft tissue coverage to protect the thoracic viscera. We define massive chest wall defects as larger than two-thirds of the chest wall. For such defects, classic flaps like the omentum, latissimus dorsi and anterolateral thigh flaps are usually insufficient. In our patient, a bilateral total mastectomy for locally advanced breast cancer resulted in a massive chest wall defect (40 by 30 cm). Soft tissue coverage was achieved with a combined anterolateral–lower medial thigh flaps. Revascularization of the anterolateral thigh and lower medial thigh components was via the internal mammary and thoracoacromial vessels, respectively. Post-operative recovery was uneventful and the patient received adjuvant chemoradiotherapy in a timely manner. The total follow up was 24-months. We illustrate the novel use of the lower medial thigh territory in extending the size of the anterolateral thigh flap to reconstruct massive chest wall defects.
INTRODUCTION
Extensive soft tissue defects of the chest wall after oncologic resection pose a great challenge to the reconstructive surgeon. It is critical to achieve durable soft tissue coverage of the chest wall for visceral coverage and facilitation of adjuvant therapy. However, in cases of massive chest wall defects (which we define as larger than two-thirds of the anterior chest wall), classic reconstructive options like the omentum or latissimus dorsi (LD) may not provide sufficient soft tissue coverage. A large flap may be obtained from the abdomen to reconstruct the chest wall; however, the resultant donor site morbidity is significant.
The anterolateral thigh (ALT) flap is the workhorse flap to reconstruct large defects in the head and neck, lower limb and thoracic wall. The anteromedial (AMT) thigh flap has been utilized as a conjoint flap with the ALT to reconstruct large chest wall defects [1]. We describe the successful use of the lower medial thigh (LMT) flap as a novel alternative in extending the ALT flap territory.
CASE REPORT
A 53-year-old lady with bilateral locally advanced breast carcinoma (LABC) (Fig. 1A) underwent bilateral total mastectomy with axillary clearance. The right pectoralis major (PM) muscle was resected, exposing the second to fourth ribs. Oncological extirpation combined with soft tissue retraction resulted in a massive defect of the anterior chest wall measuring 40 by 30 cm (Fig. 1B).
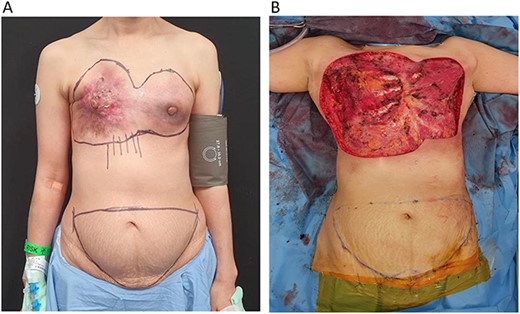
(A) 53-year-old patient with locally advanced breast carcinoma affecting both breasts. (B) Bilateral total mastectomy for locally advanced breast cancer resulted in a massive chest wall defect with exposed ribs.
Our initial reconstructive plan using bilateral abdominally based flaps (Transverse rectus myocutaneous (TRAM) flap) was unsuitable as donor site closure would be challenging with a narrow skin bridge. An ALT–AMT flap would have afforded sufficient soft tissue cover [1]. However, the absence of AMT perforators precluded its use. We identified a perforator supplying the lower medial thigh arising directly from the superficial femoral artery. The ALT flap was raised together with the LMT flap as a combined musculocutaneous flap measuring 34 by 17 cm. The great saphenous vein (GSV) was preserved within the flap (Fig. 2).
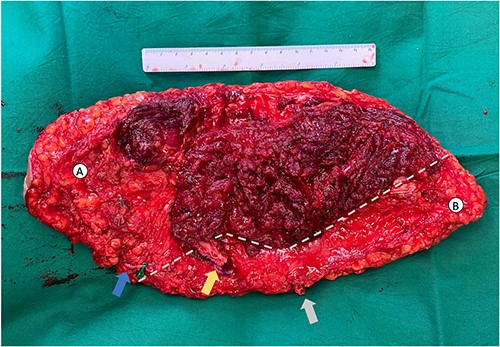
Novel anterolateral thigh–lower medial thigh (ALT–LMT) flap. (A) Denotes the ALT territory whilst (B) denotes the LMT territory, separated by a grey perforated line. The ALT pedicle is denoted by the yellow arrow and the LMT pedicle is denoted by the grey arrow. The GSV was included in the flap to improve venous drainage of this large territory flap (blue arrow).
The descending branch of the lateral femoral cutaneous vessels (dbLFCV) and LMT pedicle were anastomosed to the internal mammary vessels and thoracoacromial vessels, respectively in an end-to-end fashion. Venous drainage was augmented by anastomosing the distal end of the GSV to the right cephalic vein in a turn-down fashion (Fig. 3). The donor site was skin grafted.
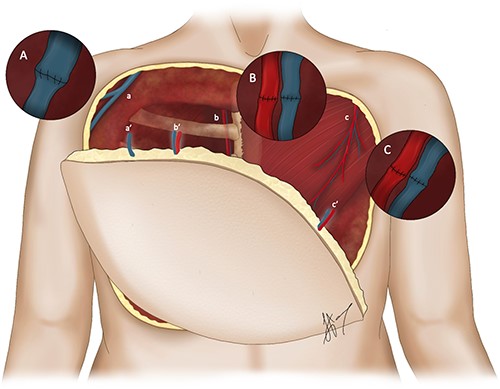
Illustration depicting the orientation of the novel ALT–LMT flap overlying the defect with the corresponding anastomoses. (A) Denotes the end-to-end anastomosis between the great cephalic vein (a) and the GSV (a’). (B) Denotes the end-to-end anastomosis between the internal mammary vessels (b) and the descending branch of the lateral femoral cutaneous vessels (b’). (C) Denotes the end-to-end anastomosis between the thoracoacromial vessels (c) and LMT pedicle (c’) respectively in an end-to-end fashion.
The chest wall and donor site (Figs 4A and B and 5) healed uneventfully. The patient commenced adjuvant chemoradiotherapy on time. The follow up was 2 years.
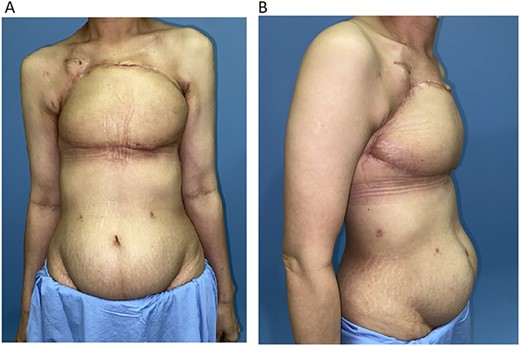
(A) 1-month post chest wall reconstruction with the ALT–LMT flap (AP view). (B) 1-month post chest wall reconstruction with the ALT–LMT flap (lateral view).
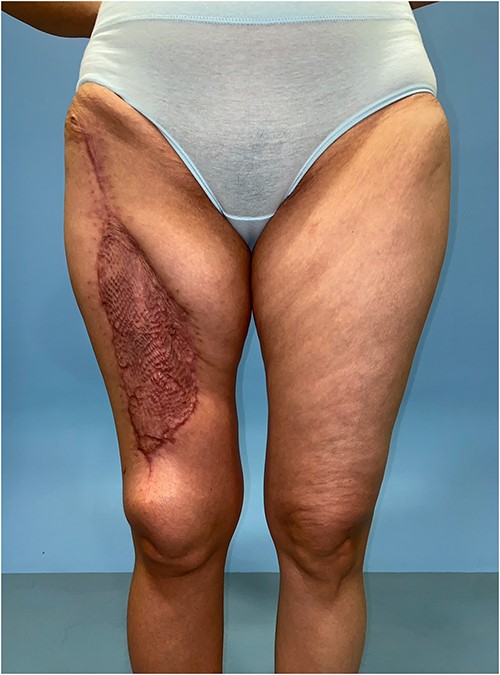
Healed donor site of the ALT–LMT flap with minimal amount of contour deformity and no functional impairment to gait and knee stability.
DISCUSSION
The treatment of LABC commonly involves chemoradiotherapy. It is important to provide well-vascularized soft tissue coverage to facilitate adjuvant therapy in a time-sensitive fashion. But the challenge lies in finding the appropriate reconstructive option for LABCs with extensive chest wall involvement.
Locoregional options like the PM, LD and the vertical rectus abdominis (VRAM) flap are traditionally employed for soft tissue defects involving less than half of the anterior chest wall. For larger defects, the aforementioned flaps can be utilized with some modification. Skeletonization of the thoracodorsal pedicle and ligation of the serratus branches allows greater mobility and advancement of the LD flap [2]. An extended or obliquely modified VRAM flap provides a longer reach and greater flap volume [3]. Free flaps such as the LD, omentum, TRAM and ALT should be considered when regional options are insufficient. However, as the defect spanned the entire horizontal and vertical length of the anterior chest wall for our patient (Fig. 1B), these traditional options were not suitable and bilateral free flaps posed high donor site morbidity risks.
A bilateral LD flap would require multiple intraoperative repositioning. The drawbacks of the omental flap include difficulty in predicting flap size and potential intra-abdominal morbidity [4]. A bilateral abdominally based flap would typically provide sufficient coverage. However, as the vertical length of the defect extended to the xiphisternum, closure of the abdominal donor site would be difficult on a narrow intervening skin bridge. Literature studies on reconstructive options for massive chest wall defects (more than two-thirds) are limited [5]. For such cases, extension beyond classically described free flap territories is required to balance soft tissue requirement and donor site complications. The ALT flap is preferred for this reason.
The dimensions of the ALT flap can be extended by recruiting the AMT or musculocutaneous tensor fascia lata territory [6]. In the absence of perforators from these territories, the LMT flap, also adjacent to the ALT, is a viable alternative. The LMT flap is supplied by perforators from the superficial femoral artery. Due to a rich anastomotic network between the descending genicular artery and the SFA, a large skin paddle can be recruited based on the LMT perforator alone [7]. We were able to reconstruct a massive chest wall defect with an ALT–LMT flap. Though intraoperative indocyanine green angiography showed adequate perfusion to the flap via the dbLFC vessels, additional microanastomosis to the LMT pedicle was performed to safeguard perfusion to this large flap. Anastomoses to the remnant pedicle of the resected PM were performed in our case. To mitigate against venous congestion, the GSV was incorporated in the flap and anastomosed to the right cephalic vein.
Large flaps have been previously described by combining multiple vascular territories in the groin and thorax. A large groin-based flap relies on the close relationship between the superficial circumflex iliac and the superficial inferior epigastric systems [8]. However, the bilobed skin paddle and short pedicle makes inset difficult and require inter-positional vein grafts [9]. The close proximity of the chest wall defect may render donor site closure difficulties in the chimeric LD–serratus anterior flap [10].
Skin grafting is required for the ALT–LMT flap donor site and a degree of contour deformity may ensue (Fig. 5). However, our patient did not encounter functional issues as knee extension and stability were preserved. She ambulated on post-operative Day 2 and did not report any weakness during squatting and climbing staircases. Unlike the AMT perforator, the LMT perforator takes a direct septocutaneous course between the sartorius and vastus medialis. There is minimal intramuscular dissection and thus no disruption to the rectus femoris.
CONCLUSION
The free ALT–LMT flap is a novel large territory flap that can be reliably used to reconstruct massive chest wall defects.
DETAILS OF EARLIER PRESENTATION
NA.
LISTING OF EACH AUTHOR’S ROLE/PARTICIPATION IN THE AUTHORSHIP OF THE MANUSCRIPT
All authors have made substantial contributions to all of the following:
The conception and design of the study, or acquisition of data, or analysis and interpretation of data.
Drafting the article or revising it critically for important intellectual content.
Final approval of the version to be submitted.
Statement of institutional board approval and/or statement of conforming to Helsinki Declaration: This study did not require any of the above.
FINANCIAL DISCLOSURE OR SUPPORT
There are no sources of funding for this work. The authors have no financial interests or financial disclosures to make. The authors have no conflicts of interest or commercial associations to declare.
ACKNOWLEDGEMENTS
There is no financial support or personal assistance of the work being published to declare.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY STATEMENT
Any required links or identifiers for our data are present in the manuscript as described.



