-
PDF
- Split View
-
Views
-
Cite
Cite
Sarah Aldosari, Bassam Altalhi, Nesreen Albisher, Shamayel Mohammed, Alaa Alhazmi, Saif Alsobhi, Castleman disease: a case report of an unusual adrenal incidentaloma and literature review, Journal of Surgical Case Reports, Volume 2023, Issue 5, May 2023, rjad248, https://doi.org/10.1093/jscr/rjad248
Close - Share Icon Share
Abstract
Adrenal incidentaloma (AI) is an incidental detection of an adrenal mass on an image not performed for a suspected adrenal problem. AI has become a commonly encountered lesion that requires further investigations for evidence of hormonal hypersecretion or malignancy potential. According to guidelines, surgical intervention is the standard of care for unilateral AI. We report on a case of a 64-year-old female who presented with a nonfunctional adrenal mass associated with compressive symptoms, which was revealed to be a mixed hyaline vascular and plasma cell variant Castleman disease (CD) after surgical resection. Although hyaline vascular variant and plasma cell variant of CD has been identified in adrenal glands, this is the first report of a mixed hyaline vascular and plasma cell variant in an adrenal mass.
INTRODUCTION
According to the National Institutes of Health (NIH), the term adrenal incidentaloma (AI) describes an inadvertent finding of an inapparent adrenal mass in the course of diagnostic testing for a condition not related to the adrenal [1]. When an adrenal mass is discovered incidentally, it is imperative to request complete investigations including hormonal assay (Table 1) and radiological images. The majority of AI present as nonfunctional adrenal lesions (85%), while a functional lesion can have the ability to produce one or a combination of hormone/s. Thus, assessing the functional status of an adrenal lesion is considered essential in the workup of AI to tailor the management.
| Disease . | Screening test/s . | Confirmatory test/s . |
|---|---|---|
| Pheochromocytoma | 24 h urine fractionated catecholamine or | NA |
| Plasma free-catecholamine | ||
| Primary Hyperaldosteronism | Plasma Aldosterone concentration and | Saline suppression test or |
| Plasma Renin activity and | Fludrocortisone suppression test or | |
| Sodium and Potassium serum level | Captopril suppression test | |
| Cushing’s Syndrome | 24 h urinary free cortisol or | Adrenocorticotropic hormone (ACTH) level |
| Midnight salivary cortisol assay or | ||
| Overnight 1 mg dexamethasone suppression test |
| Disease . | Screening test/s . | Confirmatory test/s . |
|---|---|---|
| Pheochromocytoma | 24 h urine fractionated catecholamine or | NA |
| Plasma free-catecholamine | ||
| Primary Hyperaldosteronism | Plasma Aldosterone concentration and | Saline suppression test or |
| Plasma Renin activity and | Fludrocortisone suppression test or | |
| Sodium and Potassium serum level | Captopril suppression test | |
| Cushing’s Syndrome | 24 h urinary free cortisol or | Adrenocorticotropic hormone (ACTH) level |
| Midnight salivary cortisol assay or | ||
| Overnight 1 mg dexamethasone suppression test |
| Disease . | Screening test/s . | Confirmatory test/s . |
|---|---|---|
| Pheochromocytoma | 24 h urine fractionated catecholamine or | NA |
| Plasma free-catecholamine | ||
| Primary Hyperaldosteronism | Plasma Aldosterone concentration and | Saline suppression test or |
| Plasma Renin activity and | Fludrocortisone suppression test or | |
| Sodium and Potassium serum level | Captopril suppression test | |
| Cushing’s Syndrome | 24 h urinary free cortisol or | Adrenocorticotropic hormone (ACTH) level |
| Midnight salivary cortisol assay or | ||
| Overnight 1 mg dexamethasone suppression test |
| Disease . | Screening test/s . | Confirmatory test/s . |
|---|---|---|
| Pheochromocytoma | 24 h urine fractionated catecholamine or | NA |
| Plasma free-catecholamine | ||
| Primary Hyperaldosteronism | Plasma Aldosterone concentration and | Saline suppression test or |
| Plasma Renin activity and | Fludrocortisone suppression test or | |
| Sodium and Potassium serum level | Captopril suppression test | |
| Cushing’s Syndrome | 24 h urinary free cortisol or | Adrenocorticotropic hormone (ACTH) level |
| Midnight salivary cortisol assay or | ||
| Overnight 1 mg dexamethasone suppression test |
Radiological studies in diagnosing AI depend on the imaging modality and its resolution. Computed tomography (CT) and magnetic resonance imaging (MRI) have proven to be a reliable means to diagnose AI and delineate the nature of the lesion. Another imaging modality is NP-59 adrenal scintigraphy, a functional study for the assessment of disease activity. NP-59 has shown its effectiveness in localizing the lesion (unilateral vs. bilateral) [2, 3]. The size of the lesion has been considered indicative of possible malignancy. In a meta-analysis, although the risk of malignancy increased with a tumor size measured ˃4 cm, the size alone has low specificity to differentiate a benign from a malignant lesion [3]. Other imaging properties of AI that have been described to help distinguish between benign and malignant lesions are shape, border and heterogeneity.
The published guidelines consensus that surgical intervention is the standard of care for a unilateral AI that poses unambiguous hormonal activity and with suspected malignancy, whereas nonfunctional benign AI does not require surgical intervention [1, 4]. NIH has also recommended surgical intervention for an AI that is ˃6 cm regardless of hormonal activity [1], and some evidence proposed a lower cut-off for surgical intervention [5].
One of the rare differential diagnoses of AI is Castleman disease (CD), a rare lymphadenopathy disorder with different subtypes. Based on radiological and clinical manifestation, it can be classified as unicentric (localized) and multicentric (generalized). Pathologically, it is classified as hyaline vascular type, plasma cell type or mixed type. There is scant knowledge about the etiology of CD; however, various shreds of evidence postulated that it occurs due to impairment in immunoregulation, causing proliferation of B lymphocytes and plasma cells in lymphoid tissues. The median age of patients was reported to be 43 years of age with a female predilection. In total, ⁓70% of CD is present in the thoracic area and rarely in the adrenal/ pararenal region. The final diagnosis of CD is based on histological and immunohistochemistry, and the primary treatment for CD presented as an adrenal mass is an adrenalectomy.
CASE PRESENTATION
A 64-year-old Saudi female was referred to our clinic with an incidental finding of an adrenal mass by CT scan. The patient is a known case of long-standing resistant hypertension (High reading with fluctuation) on valsartan/amlodipine/hydrochlorothiazide, and later switched to verapamil and losartan. Recently, the patient started to complain of generalized body pain, for which she sought medical attention. Multiple images were requested, and an abdominal CT scan revealed an adrenal mass measured 6 cm, thus the patient was referred to our hospital for further management.
Upon presentation, the patient revealed that she has a positive history of multiple episodes of palpitation and headache for the past years. Physical examination demonstrated an obese female (body mass index: 35) with a blood pressure of 144/92, and no other abnormalities were noted. The patient underwent investigation as a case of AI. The results of laboratory studies, including complete blood count, renal profile and thyroid function test were within the reference range. Hormonal assay was obtained and was all normal, consistent with a nonfunctional adrenal mass (Table 2).
| Hormone . | Result . | Interpretation . |
|---|---|---|
| Aldosterone | <0.09 ng/dL | Low |
| Renin | <0.5 Mu/L | Low |
| Cortisol | 373 nmol/L | Normal |
| Cortisol post dexamethasone suppression test | 19 nmol/L | Normal |
| ACTH post dexamethasone suppression test | 3 ng/L | Low |
| Testosterone | 12 nmol/L | Normal |
| Dehydroepiandrostenedione | 2.75 umol/L | Normal |
| Metanephrine | <0.06 umol/L with excretion of <0.21 umol/day | Normal |
| Normetanephrine | 0.23 umol/L with excretion of 0.82 umol/day | Normal |
| Methoxytyramine | 0.11 umol/L with excretion of 0.39 umol/day | Normal |
| Hormone . | Result . | Interpretation . |
|---|---|---|
| Aldosterone | <0.09 ng/dL | Low |
| Renin | <0.5 Mu/L | Low |
| Cortisol | 373 nmol/L | Normal |
| Cortisol post dexamethasone suppression test | 19 nmol/L | Normal |
| ACTH post dexamethasone suppression test | 3 ng/L | Low |
| Testosterone | 12 nmol/L | Normal |
| Dehydroepiandrostenedione | 2.75 umol/L | Normal |
| Metanephrine | <0.06 umol/L with excretion of <0.21 umol/day | Normal |
| Normetanephrine | 0.23 umol/L with excretion of 0.82 umol/day | Normal |
| Methoxytyramine | 0.11 umol/L with excretion of 0.39 umol/day | Normal |
| Hormone . | Result . | Interpretation . |
|---|---|---|
| Aldosterone | <0.09 ng/dL | Low |
| Renin | <0.5 Mu/L | Low |
| Cortisol | 373 nmol/L | Normal |
| Cortisol post dexamethasone suppression test | 19 nmol/L | Normal |
| ACTH post dexamethasone suppression test | 3 ng/L | Low |
| Testosterone | 12 nmol/L | Normal |
| Dehydroepiandrostenedione | 2.75 umol/L | Normal |
| Metanephrine | <0.06 umol/L with excretion of <0.21 umol/day | Normal |
| Normetanephrine | 0.23 umol/L with excretion of 0.82 umol/day | Normal |
| Methoxytyramine | 0.11 umol/L with excretion of 0.39 umol/day | Normal |
| Hormone . | Result . | Interpretation . |
|---|---|---|
| Aldosterone | <0.09 ng/dL | Low |
| Renin | <0.5 Mu/L | Low |
| Cortisol | 373 nmol/L | Normal |
| Cortisol post dexamethasone suppression test | 19 nmol/L | Normal |
| ACTH post dexamethasone suppression test | 3 ng/L | Low |
| Testosterone | 12 nmol/L | Normal |
| Dehydroepiandrostenedione | 2.75 umol/L | Normal |
| Metanephrine | <0.06 umol/L with excretion of <0.21 umol/day | Normal |
| Normetanephrine | 0.23 umol/L with excretion of 0.82 umol/day | Normal |
| Methoxytyramine | 0.11 umol/L with excretion of 0.39 umol/day | Normal |
An MRI revealed a right adrenal mass with an avid enhancement of central cystic changes, and the mass was abutting the medial border of segments 8 and 7 of the liver without invasion (Fig. 1). The final impression was an incidental nonfunctional right adrenal mass with a clinical and radiological characteristic of pheochromocytoma.
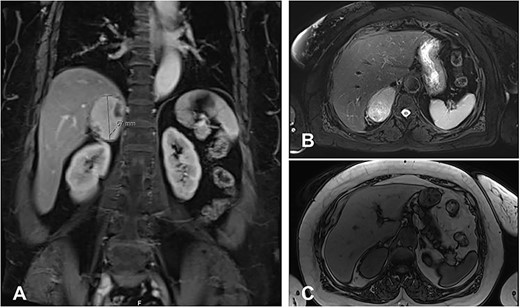
MRI Abdomen with and without IV contrast. Right adrenal mass demonstrating high T2 and low T1, with cystic changes. (A), an axial T2 weighted MRI shows a hyperintense right adrenal mass with cystic changes, abutting the liver without invasion. (B), a cross-section T2 weighted MRI shows a hyperintense large right adrenal mass. (C), cross-section T1 weighted MRI shows a large right adrenal mass characterized by hypointernse lesion compared with hepatic parenchyma.
The patient underwent right laparoscopic adrenalectomy with a transperitoneal approach. A dark yellow tumor measuring 11 × 7 × 3.5 cm was removed en bloc (Fig. 2). Postoperative convalescence was uneventful and the patient was discharged home after 2 days, with discontinuation of antihypertensive medications. Microscopic examination revealed a mixed hyaline vascular and plasma cell variant type of Castleman lymphadenopathy (Figs 3 and 4). Immunohistochemistry showed a mixed pattern of CD20 and CD3, IgD that highlighted the expanded mantle zones and CD138-positive plasma cells that showed polytypic expression of Kappa and lambda immunoglobulin light chains. Immunostaining for Human herpesvirus 8 (HHV-8) was positive. A diagnosis of a unicentric mixed variant of Castleman lymphadenopathy was made.
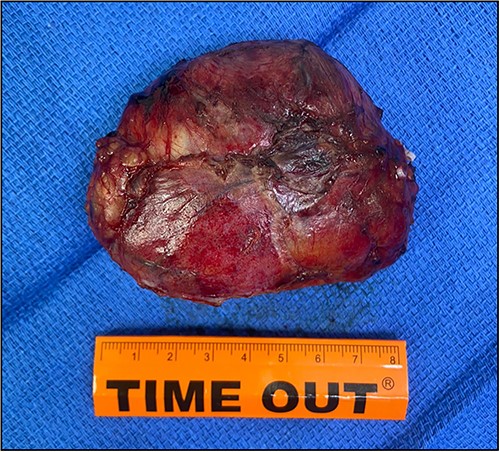
An adrenal mass with cystic and vascular-rich component that was removed surgically, measuring ⁓11 cm.
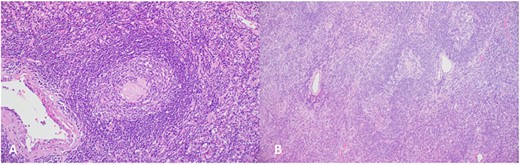
(A), Regressed germinal center shows hyalinized blood vessels, dendritic cells and mantle zone forming an ‘onion skin’ arrangement; (B), the hyalinized blood vessels together with the atretic follicles and concentric mantle zone import a ‘lollipop’ appearance.
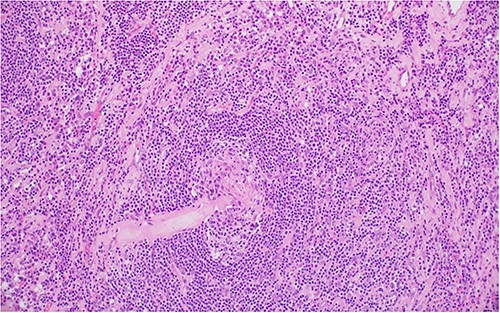
Two germinal centers fused and surrounded by one mantle zone—‘twinning’.
DISCUSSION
CD is a rare benign lymphoproliferative disorder initially described by Benjamin Castleman, and after that several case series were reported [6, 7]. The etiology and pathogenesis of CD are still to be elucidated and further investigations are warranted to understand the disease. Diagnosing CD is solely based on histopathological assessment [8]. Two histopathological variants of CD were identified in the literature [9, 10]. The initial histopathological variant of CD was the hyaline vascular type, which includes 90% of the reported cases [7, 9, 11, 12]. Later, a distinct variant was identified by Castleman in 1971 and termed plasma-cell type [10]. A mixed variant also exists but is rarely reported [9, 10].
The classification of CD is based on the number of the involved lymph nodes: unicentric CD (UCD) and multicentric CD (MCD). UCD is defined as a single lymphadenopathy. Whereas MCD is characterized by generalized lymphadenopathy, and it commonly presents with systematic symptoms [9, 11]. Although UCD occurs commonly in the mediastinum (70%), other locations were also reported such as the neck, axilla, lung, mesentery, retroperitoneum and pelvis [7, 9, 11]. Frequently, UCD is diagnosed incidentally since it behaves in an indolent manner. Unlike MCD, systemic constitutional symptoms are rarely encountered in UCD, and it mostly present due to symptoms secondary to compression [8].
Management of UCD depends on the location and resectability of the lymph node. Although different management approaches have been proposed, surgical resection is the gold-slandered and widely accepted method for a resectable UCD [8, 10, 12]. This was further supported by the recently published evidence-based guidelines [8]. Also, Surgical resection is highly recommended for a resectable UCD to alleviate compression symptoms and to rule out malignant potential since UCD-related malignancy risk is unclear [13, 14]. Sometimes, the size and location of UCD make it surgically challenging, and further discussion in a multidisciplinary meeting is warranted. Meanwhile, no systematic studies were published to analyze the optimal management of an unresectable UCD. Consensus opinions suggested that asymptomatic UCD could be monitored and managed non-surgically since it grows slowly and as long there are no threatened adjacent structures by the compression [8]. Monoclonal antibodies and radiotherapy were introduced to reduce the size of unresectable UCD followed by surgical evaluation [8]. Overall, UCD was shown to carry a good prognosis. A systematic review reported that the 3-year free-disease survival rate was 95% [15].
CONCLUSION
To our knowledge, we report a never-described mixed variant of CD as an adrenal mass. CD is an uncommon and unique entity with different histopathological variants. Preoperative investigations are likely to be inconclusive and can impact a patient’s outcome. Thus, surgical resection provides a clear specimen for a definitive diagnosis confirmed by histology and immunohistochemistry.
INFORMED CONSENT
Verbal informed consent was obtained from the patient for publication of this case report. The work was written in line with the SCARE criteria [16].
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING
None declared.
ETHICAL APPROVAL
The approval of the ethics committee for the retrospective analysis of a clinical case report is not required in our institution.



