-
PDF
- Split View
-
Views
-
Cite
Cite
Robert Reis Skylis, Gabriela Bueno de Souza, Ricardo Camillo de Almeida, Rogério Aparecido Dedivitis, Pleomorphic adenoma in tonsil, Journal of Surgical Case Reports, Volume 2023, Issue 5, May 2023, rjad261, https://doi.org/10.1093/jscr/rjad261
Close - Share Icon Share
Abstract
Salivary tumors that involve the tonsil are rare, representing 0.5% of salivary neoplasms. Most tumors arise from the parotid glands. Rare studies have been reported in the literature regarding pleomorphic adenomas affecting the tonsil. Patients are usually asymptomatic and present with a slow-growing, mobile and painless mass. Radiological examinations are usually unable to differentiate benign from malignant tumors in most cases. There is low recurrence with complete excision of the tumor. The prognosis is excellent except in the rare cases of malignant transformation. This report describes the management of a case of pleomorphic adenoma affecting the palatine tonsils and the difficulty regarding the histopathological diagnosis.
INTRODUCTION
Salivary neoplasms, regardless of the histological type, primarily affect the parotid gland, in ~70% of cases. Other types such as the submandibular gland and palate represent 8.4 and 8% respectively, while rarer sites such as the tonsils (0.5%), ethmoid sinuses (0.3%) and nasopharynx (0.3%) can also be found and be affected but to a lesser extent [1].
Pleomorphic adenoma (PA) is the most common benign neoplasm, accounting for 40–70% of all tumors. The occurrence of PA can affect any age group, but is especially common in the third and fourth decades of life, with a predominance among women. Intraoral lesions occur mainly on the hard palate, followed by the upper lip and buccal mucosa [2].
The main histopathological differential diagnosis of PA is myoepithelioma. The main difference between them is the presence of myoepithelial cells throughout the extension of the myoepithelioma, and the absence or rarity of ductal formations. On the other hand, in PA, myoepithelial cells, in most cases, are present in varied numbers, and ductal formations are present in large numbers [3]. Both cases of tumors have a good prognosis if treated with complete surgical excision, since the recurrence of salivary neoplasms increases when the tumor is incompletely excised [4].
We report an unusual case of salivary gland tumor in the palatine tonsil and the histopathological differential between PA and myoepithelioma.
CASE REPORT
A female patient, 72 years old, sought care reporting intermittent dysphonia for 5 months. She claimed to have hypothyroidism and hypertension, using continuous medication. She denied other symptoms such as weight loss, dyspnea, dysphagia and fever. Two external laryngoscopies had been performed, and a vegetating lesion was found on the left lateral wall of the oropharynx, measuring 3.5 × 2.5 cm with mass effect merging with the left palatine tonsil (Fig. 1). Nuclear magnetic resonance revealed a mixed lesion, containing a cyst with a thick content measuring 23 × 22 × 24 mm (Fig. 2). Given this finding, the chosen procedure was resection by suspension laryngoscopy with the use of CO2 laser. Mass resection of the left tonsil was performed successfully and uneventfully, removing a solid, fibroelastic, 3 × 2 cm mass. Laryngoscopy after 1 month showed good healing, with no evidence of remaining lesions. After histopathological analysis, the tumor was benign and well-delimited (Fig. 3). The main diagnostic hypothesis was myoepithelioma of the oropharynx; however, after analysis of the immunohistochemical evaluation of the specimen, the result was inconclusive between myoepithelioma or PA, favoring the second.
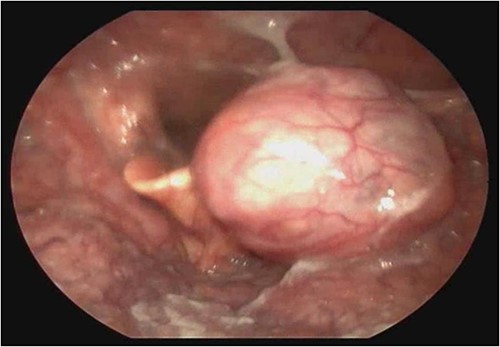
Laryngoscopy showing a vegetating lesion on the lateral wall of the oropharynx.
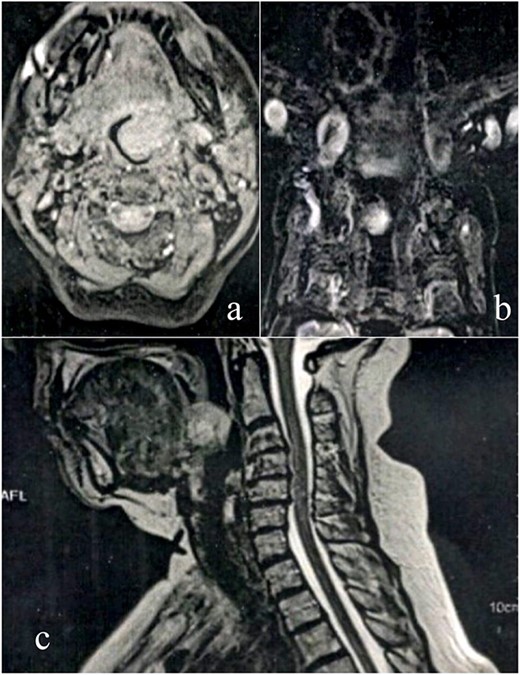
MRI scan shows tumor extension. (a) Axial cranioencephalic section. (b) Coronal section of the chest. (c) sagittal section.
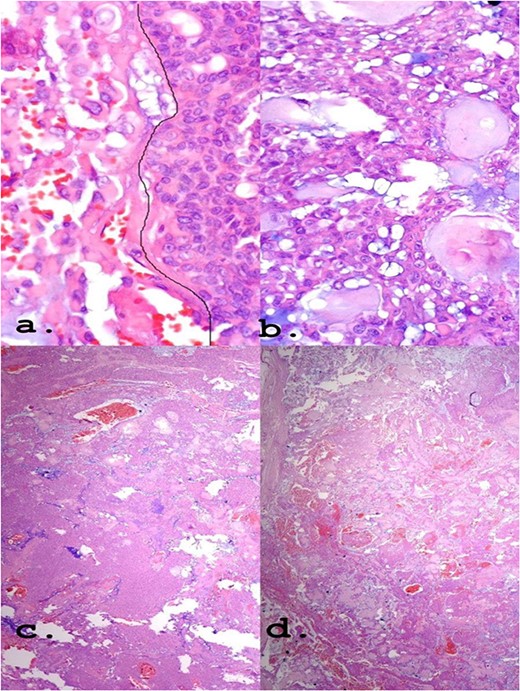
(a) Epithelioid area on the left. (b) Area of triple epithelial cell phenotype (plasmacytoid, myoepithelial and ductal). (c) Highly cellular lesion with a tendency to cellular monomorphism and scarce stromal component. (d) Very limited neoplasm—green India ink—margin surgical HE, ×40.
DISCUSSION
In this case report, the age of 72 years and the primary site of tumor growth do not match the usual statistics of a common PA. Location in the tonsil is rare, representing only 0.5% of cases [1]. As it is an oligosymptomatic lesion, the tumor was diagnosed with a large volume.
The need for a correct diagnosis is of great importance to exclude malignancy and stipulate recurrence according to histological type. PA, for example, is the type with the highest risk of malignancy (5–15%) and recurrence when compared to others, which rarely become malignant. Histopathological diagnosis can be challenging, and there are no biomarkers that can predict malignant potential or recurrence. Although some studies indicate that p63 is significantly decreased in carcinomas ex PA [5, 6].
PA is commonly represented by having wide architectural and cytomorphological diversity. It has cells capable of differentiating into epithelium and myoepithelium, as well as into mesenchymal cells of the chondroid, myxoid and even bone types. The epithelial cells, arranged in an arrangement of cords or leaflets, can be ductal or non-ductal, and the presentation can be predominant with epithelial richness or have only foci of epithelium, thus being variable [7].
Regarding the differentiation between myoepithelioma and PA, the analysis of the presence of ducts is a plausible way of distinguishing since these are rare in myoepitheliomas. Although less frequent, myoepithelioma may present myxoid or chondroid stroma, which bears a resemblance to PA. Some other common findings may make the diagnosis difficult, such as the abundance of myoepithelium and areas with pure myoepithelial cells [5, 8].
Regarding morphological differentiation, myoepithelioma has a solid, tan, yellow appearance, with a bright cut on the surface, and the PA usually appears as a single, firm, mobile and well-circumscribed mass. On the cut surface, they vary from light brown to gray, with or without cartilaginous features. Degenerative and cystic changes can be observed [9, 10].
The positivity of markers such as p63, myoepithelial cell markers and AE1/AE3, epithelial cells, found in immunohistochemistry (Fig. 4) may also be present in myoepitheliomas but in a lower percentage, ranging from 17 and 44% [8]. However, in this report, a large number of plasmacytoid epithelial cells and mainly ductal formations are superimposable characteristics of the PA, despite the scarce stromal component and foci of myoepithelium.
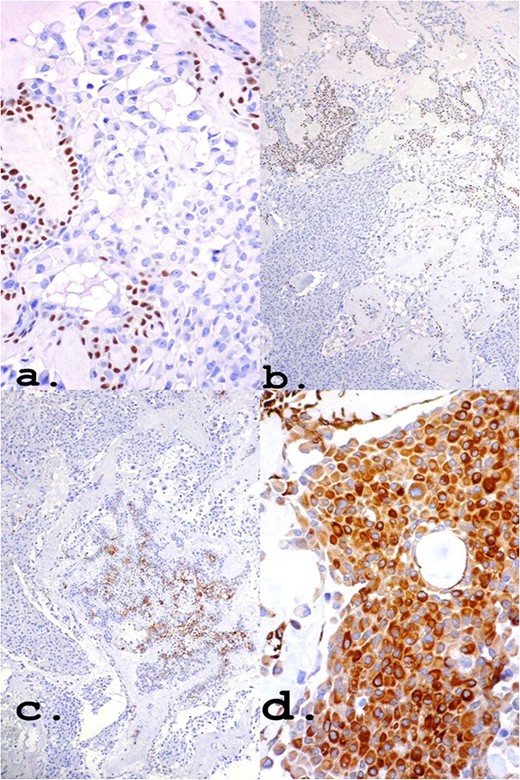
Own nuclear marker of myoepithelial cells (a) and (b): p63 ×40 and ×10, respectively. Markers for epithelial cells (c) and (d): CK7 ×10 and Pancytokeratin (AE1–AE3) ×40, respectively.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
DATA AVAILABILITY
We declare that all data used in the preparation of this manuscript are available upon request to the authors.



