-
PDF
- Split View
-
Views
-
Cite
Cite
Haley Feltracco, Abraham J Matar, Savannah A Smith, Catherine Blair, Juan M Sarmiento, Surgical management of a giant hepatic metastasis from a cranial meningioma 10 years after resection, Journal of Surgical Case Reports, Volume 2023, Issue 5, May 2023, rjad238, https://doi.org/10.1093/jscr/rjad238
Close - Share Icon Share
Abstract
Meningiomas are the most common type of primary brain tumor; they have a low risk for extracranial metastases, which are primarily associated with increased tumor grade. Hepatic metastases from cranial meningiomas are extremely rare, with only a paucity of cases reported in the literature and no standardized approach to management. Herein, we report a case of an incidentally discovered giant (>20 cm) metastatic meningioma to the liver treated with surgical resection 10 years following resection of a low-grade cranial meningioma. This report also highlights the use of (68Ga) DOTATATE PET/CT as the diagnostic imaging modality of choice when evaluating for meningioma metastases. To our knowledge, this report describes the largest hepatic metastasis from a cranial meningioma to undergo surgical resection in the literature.
INTRODUCTION
Meningiomas are the most common primary central nervous system tumor. Despite this, extracranial metastases of meningiomas are exceptionally rare, occurring in only 0.1% of all meningioma cases [1–4]. Cranial meningiomas are categorized into three distinct histopathological grades—benign (WHO Grade I), atypical (WHO Grade II) and anaplastic (WHO Grade III)—with worse prognosis associated with increasing grade [5, 6]. The risk of metastatic disease is based on inherent tumor characteristics, including cellularity, cellular heterogeneity, mitotic rate, nuclear pleomorphism, tumor necrosis and lymphovascular invasion, all of which are more commonly associated with atypical and anaplastic tumors [2–4, 6]. The route of dissemination remains a topic of debate, with hematogenous spread, particularly via the paravertebral and jugular venous systems, thought to be the most common route [5, 7, 8]. The most common sites of metastases include lungs, pleura, mediastinum, axial bones and lymph nodes [3]. Hepatic metastases of cranial meningiomas are exceedingly rare, accounting for roughly 3% of all extracranial metastases, and the majority are discovered at a size <10 cm [5]. Herein, we present an unusual case of a patient who presented with a giant >20 cm hepatic metastasis of a WHO Grade 1 cranial meningioma more than 10 years following cranial resection.
CASE REPORT
The patient is a 61-year-old female with a history of cervical cancer diagnosed in 2002 treated surgically and subsequently treated with systemic chemo and radiation for a local recurrence, currently in remission. In 2011, she presented to a separate institution with expressive aphasia, memory loss and right-sided homonymous hemianopsia. An MRI demonstrated a 6 × 7 cm left intraventricular mass. The patient underwent surgical resection with pathology consistent with a WHO Grade 1 meningioma with a mitotic rate of 2 mitoses/10 HPF. Immunohistochemical staining revealed the tumor was positive for SSTR2a, EMA and FLI1 and negative for CD34. The patient remained disease free until 2018, when an MRI revealed a cranial recurrence, and she was treated with radiation for a 3-month period.
In July 2022, the patient developed right upper-quadrant pain prompting an abdominal ultrasound, which demonstrated a large mass in the right lobe of the liver. An abdominal MRI revealed a 23 × 17 cm heterogeneous mass with mild arterial enhancement in the right lobe of the liver with mass effect on the intrahepatic inferior vena cava (IVC) (Fig. 1). An enhancing L1 vertebral lesion was also discovered on the MRI with a differential diagnosis of atypical intraosseous hemangioma versus metastatic disease. Hepatic function tests as well as tumor markers including AFP, CEA, CA-125 and CA19-9 were normal. A CT guided biopsy of the liver lesion was consistent with metastatic meningioma. Pathologic review and comparison with the initial cranial meningioma revealed a similar immune profile (positive for SSTR2a, EMA, and FL1 negative for CD34) and mitotic rate (2 mitoses/10 HPF). A (18F) FDG PET/CT scan demonstrated a large right hepatic mass with significant metabolic activity, although the lesion in L1 was not PET avid. The patient was discussed at a multidisciplinary tumor board and surgical resection was recommended given the patient’s symptomatology as well as the large size and mass effect on the IVC.
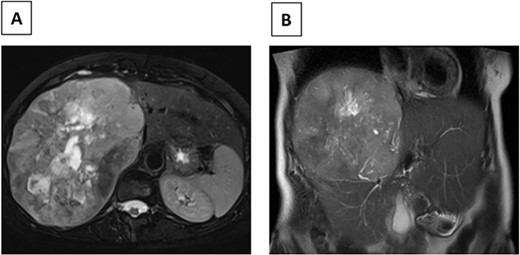
(A) Axial MRI image (T2) of R hepatic lobe mass. (B) Coronal MRI image (T2) of R hepatic lobe mass.
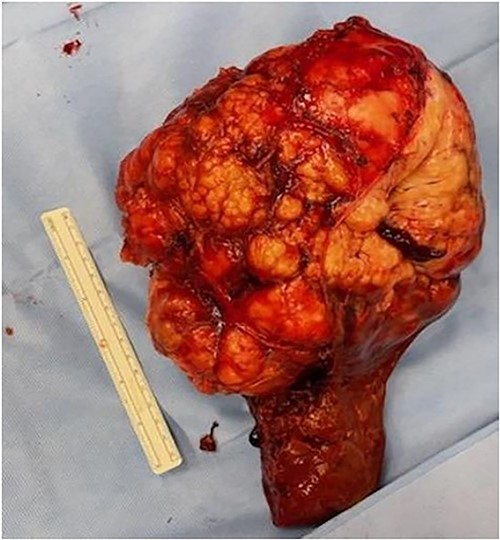
The patient underwent an exploratory laparotomy with right hepatectomy and cholecystectomy utilizing a low CVP strategy. An intrahepatic Glissonian approach was utilized and the right sided vascular inflow and outflow were transected during the parenchymal transection rather than extra-hepatically. Intraoperatively, blood loss was 750 mL, largely during parenchymal transection. A pringle maneuver was not used. The patient received intraoperative resuscitation with 1500 mL crystalloid, 500 mL 5% albumin, 1 unit of packed red blood cells (prbc) and 1 unit of autologous prbc via an intraoperative cell salvage device. Following removal of the specimen, intraoperative ultrasound was utilized to survey the remaining left liver; however, no other concerning lesions were identified. The patient was managed on the surgical floor postoperatively with an unremarkable postoperative course, requiring 1 unit of prbc on postoperative day (POD) 4 because of a hemoglobin level of 6.9 gm/dL with an appropriate and sustained response. The patient was subsequently discharged home on POD 5. The final surgical pathology was consistent with meningioma, and all surgical margins were negative > 1 cm (Fig. 2).
A (68Ga) DOTATATE PET/CT was performed 1 month postoperatively for surveillance purposes which revealed a 1 cm intense lesion in segment 4A of the liver, as well as several radiotracer avid osseous foci involving the left parietal bone, T9 and L1 vertebrae, and left proximal humerus, concerning for further metastatic disease (Fig. 3). The patient was discussed at a multidisciplinary tumor board, and the decision was for ongoing surveillance to her liver with the option for liver directed therapy should the segment 4A lesion grow as well as to proceed with radiation treatment to the vertebral lesions.
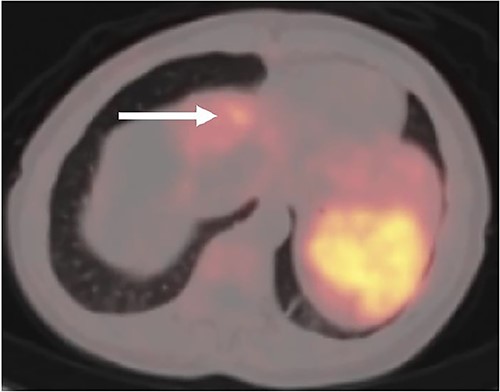
Postoperative (68Ga) DOTATATE PET/CT imaging demonstrating 1 cm intense lesion in segment 4A of the left hemiliver (white arrow).
DISCUSSION
The overall incidence of extracranial metastases of cranial meningiomas is low, and the vast majority are associated with high-grade tumors (WHO Grades 2/3), whereas metastases associated with low-grade tumors (WHO Grade 1) are rare. In one of the largest studies of cranial meningiomas, seven of 396 were classified as high grade based on histological criteria, with a metastatic rate of 43%. Among the other 389 low-grade meningiomas, the rate of metastasis was 0.76%, suggesting the incidence of metastatic disease is largely dependent on the grade of the primary tumor [9]. Other data have supported this finding, noting an overall incidence of metastasis ~0.2%, but noting a metastatic rate up to 40–45% in WHO Grade III tumors [4]. The patient we present here was initially diagnosed with a WHO Grade I cranial meningioma in 2011, suggesting a low likelihood of future metastatic disease.
In addition to the low-grade nature of the primary tumor, the hepatic location of metastatic disease in this patient is uncommon [1, 4]. Beutler et al. reported one case of a WHO Grade 1 cranial meningioma presenting 7 years after initial diagnosis with multiple liver metastases (<5.5 cm) treated with systemic therapy. Notably, complete surgical resection of the primary tumor was not achieved because of involvement of the superior sagittal sinus. In our patient, the primary tumor had undergone complete surgical resection with negative margins more than 10 years prior to presentation with a liver metastasis, although the patient had a local recurrence in the interim and was treated with radiation. Several reports have described the diagnosis of a liver mass as metastatic meningioma postoperatively on histological analysis of a surgical specimen without a preoperative biopsy [1, 7]. Obiorah et al. [1] reported a single liver metastasis (7.8 cm) diagnosed as metastatic meningioma in the absence of a known primary tumor based on histological analysis following partial hepatectomy. A postoperative MRI of the brain revealed a 1.4 cm dural mass that was resected and confirmed as a WHO Grade 1 meningioma. Similarly, Sheng et al. [7] describe a patient with a known history of cranial meningioma 4 years prior who underwent resection of 7 × 5 cm segment seven liver mass without preoperative biopsy. The diagnosis of metastatic meningioma was made after IHC demonstrated tumor cells with identical histology as the primary meningioma. Given the rare incidence of hepatic metastases of cranial meningiomas and the inability to make a definitive diagnosis based on imaging alone in our case, we elected for a preoperative biopsy, which confirmed the diagnosis.
The choice of systemic therapy in the report by Beutler et al. [4] also highlights the lack of standardized treatment approach to metastatic meningioma. The metastatic lesions in that report were confined to segments 7/8 and therefore amenable to surgical resection. Given our patient’s presentation of right upper quadrant pain, along with the massive size of the metastatic lesion occupying much of the right hepatic lobe and compression of the IVC, it was felt that surgical resection was the optimal treatment strategy. Liver directed therapy and/or systemic therapy would have been unlikely to elicit a significant treatment response. To our knowledge, our report represents the largest hepatic metastasis from a cranial meningioma to undergo surgical resection in the literature. Jenkinson et al. [10] reported a 28 × 18 cm vascular tumor replacing the right lobe of the liver in a 33-year male with a history of a parieto-occipital meningioma excised 5 years prior. Liver biopsy confirmed a morphologically identical lesion to the meningioma; however, there is no report of surgical resection. In our patient, the decision was made to forego a minimally invasive approach given the massive size of the tumor and compression of the IVC. We utilized an intraoperative cell salvage device given the proximity of the tumor to the IVC and potential for significant blood loss during the parenchymal transection.
Recent data have demonstrated the utility of (68Ga) DOTATATE PET/CT to aid in the imaging of meningiomas given that meningiomas express the somatostatin receptor that normal tissues lack [11–13]. It is possible that a pre-operative (68Ga) DOTATATE PET/CT may have helped identify the 1 cm lesion in segment 4A concerning for a satellite metastasis detected on postoperative imaging, although the signal and proximity of the dominant metastasis may have overshadowed the smaller lesion. Similar to the use of (68Ga) DOTATATE PET/CT for identification of hepatic neuroendocrine metastases, (68Ga) DOTATATE PET/CT should be considered the diagnostic imaging modality of choice when evaluating for meningioma metastases. This patient remains under close surveillance of further metastatic disease and may be a candidate for either systemic therapy or targeted liver directed therapy for the segment 4A lesion should it demonstrate interval growth. Previously reported systemic therapies currently in use in the treatment of metastatic meningioma include bevacizumab, hydroxyurea and ifosfamide—highlighting the lack of a first line treatment [4, 14–16]. Similarly, there are limited reports of liver directed therapy for hepatic metastatic meningioma. Ferguson et al. [17] reported a patient presenting with symptomatic hypoglycemia because of extensive bilobar liver metastases secondary to a cranial meningioma. The patient underwent selective arterial chemo-embolization in an effort to palliate symptoms.
CONCLUSION
The overall incidence of metastatic cranial meningioma is exceedingly rare, especially in the setting of low-grade primary tumors. Hepatic metastases from cranial meningiomas can represent both a diagnostic and therapeutic challenge given the infrequent and varying presentations. Given the infrequent nature of hepatic metastases, there is no standard treatment, although surgical resection likely provides the greatest chance for cure. Prior to surgical resection, histopathological analysis can establish a definitive diagnosis in the majority of cases, and a (68Ga) DOTATATE PET/CT is warranted to evaluate for distant sites of disease. To our knowledge, this report represents the largest hepatic metastasis from a cranial meningioma to undergo surgical resection in the literature.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
AUTHORS' CONTRIBUTIONS
A.J.M. and J.M.S. conceived of the manuscript. H.F., S.A.S. and A.J.M. wrote the manuscript. A.J.M., C.B. and J.M.S. assisted in the clinical care of the patient. A.J.M. and J.M.S. edited the manuscript.



