-
PDF
- Split View
-
Views
-
Cite
Cite
Toshihiro Kawahira, Hiroki Morimoto, A rare case of positional vertebrobasilar ischemia with the retrograde flow of the vertebral artery, Journal of Surgical Case Reports, Volume 2023, Issue 5, May 2023, rjad222, https://doi.org/10.1093/jscr/rjad222
Close - Share Icon Share
Abstract
Positional vertebrobasilar ischemia, also known as Bow hunter stroke, is typically caused by mechanical compression of the vertebral artery (VA). On the other hand, subclavian steal syndrome is incidentally detected by vertigo, syncope or loss of consciousness due to the steal phenomenon. A 61-year-old man suffered from near syncope when he turned his head to the left side. Although asymmetric arm blood pressure of the right dominant was observed, arm claudication was not detected. Computed tomographic angiography and magnetic resonance imaging revealed total occlusion of the left subclavian artery, hypoplasia of the right VA and an incomplete circle of Willis. Furthermore, carotid Doppler ultrasonic echography revealed retrograde blood flow of the left VA. It means that head rotation might occur in the left VA ischemia. An axillary-axillary bypass surgery was performed and ultrasonic echography showed anterograde effective blood flow of the left VA after the procedure.
INTRODUCTION
In the field of neurological surgery, head rotation-induced faintness, also known as Bow hunter stroke (BHS), is usually caused by insufficient blood flow of the vertebral artery (VA) [1]. Conversely, subclavian steal syndrome (SSS) is also one of the most important diseases that is sometimes identified by vertebrobasilar ischemia (VBI) like BHS in the field of vascular surgery. BHS is typically diagnosed via the VA compression; however, the symptoms of VBI from SSS is caused by the steal phenomenon of retrograde VA flow with arm claudication.
This study describes an atypical condition that contributes to near syncope as positional VBI with total occlusion of the left subclavian artery, retrograde left VA flow without steal phenomenon and hypoplasia of the right VA with an incomplete circle of Willis.
CASE REPORT
A 61-year-old man was referred to our hospital for cardiac and vascular evaluation after experiencing vertigo and near syncope for the past 2 years. His symptoms were usually triggered when he turned his neck to the left side, and his vertigo worsened at that time. He had never been diagnosed with any cardiovascular abnormalities. Clinical examination revealed no headaches or chest pains, however there was asymmetric arm blood pressure, 166/105 and 107/83 mmHg in the right and left arms, respectively. The electrocardiogram indicated a normal sinus rhythm. Chest enhanced computed tomography (ECT) revealed a long total occlusion site of the left subclavian artery, and the left VA was suspect of having retrograde blood flow from some collateral arteries (Fig. 1) Therefore, carotid Doppler ultrasonic echography (CDUS) could clarify it directly (Fig. 2). The laboratory results revealed only hyperlipidemia.
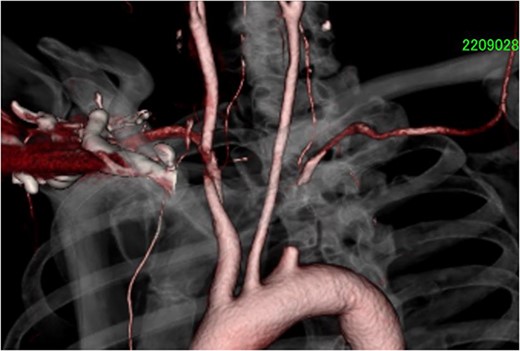
Chest ECT showed total and long occlusive lesion of the left subclavian artery. ECT, enhanced computed tomography.
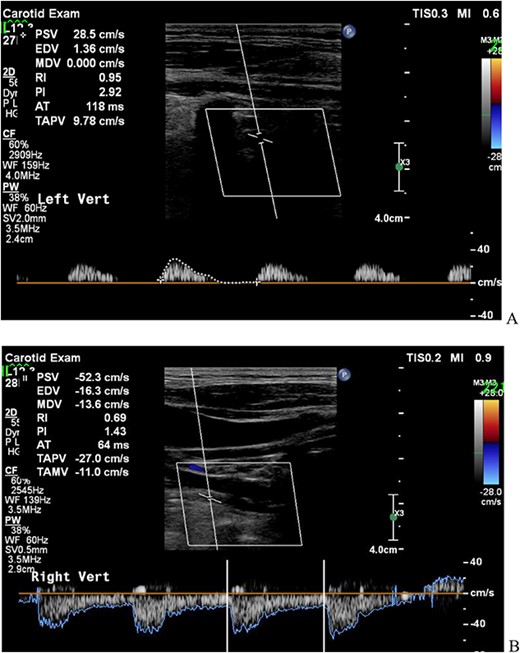
CDUS showed the retrograde blood flow of the left VA before operation (A; left VA, B; right VA). CDUS, carotid Doppler ultrasonic echography; VA, vertebral artery.
A transthoracic cardiac echocardiogram revealed that the left ventricular ejection fraction was normal. Magnetic resonance imaging (MRI) also recorded the retrograde blood flow of the VA, and lacunar infarction of the right thalamus was detected without any other infarction (Fig. 3). Furthermore, an incomplete circle of Willis was revealed by hypoplasia of the right VA without connection to the basilar artery and hypoplasia of the left posterior communicating artery (Fig. 4).
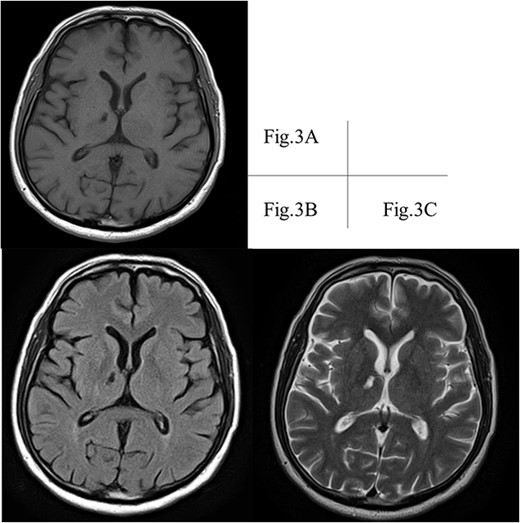
A right thalamic lacunar infarction is detected hypointense on T1-weighted image. (A) and FLAIR image (B), and hyperintense on T2-weighted image (C). FLAIR, fluid-attenuated inversion recovery; MRI, magnetic resonance imaging.
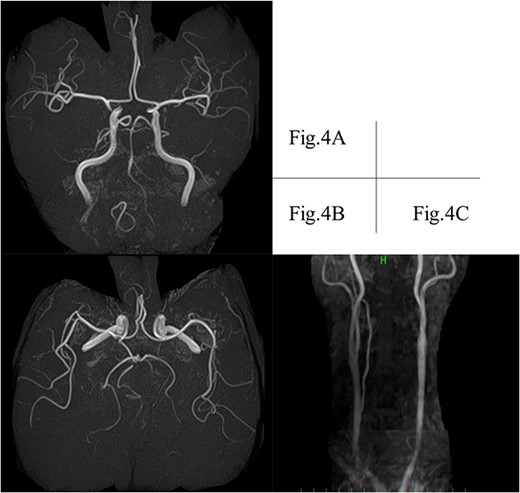
MRI depicts hypoplasia of the right VA without connection to the basilar artery. (A) Left posterior communicating artery (B) and suspected retrograde flow of the left VA (C). MRI, magnetic resonance imaging; VA, vertebral artery.
After receiving informed consent, an axillar-axillar bypass was performed under general anesthesia. The skin incision was performed to expose the bilateral axillary arteries. Initially, the right axillary artery was anastomosed endo to side by CV-5® through the skin with a 6-mm incompressible GORETEX® vascular prosthesis, followed by the left side. Before recanalisation to the left subclavian artery, the left VA was manually compressed. The CDUS could detect antegrade blood flow in the left VA at the end of the operation (Fig. 5).
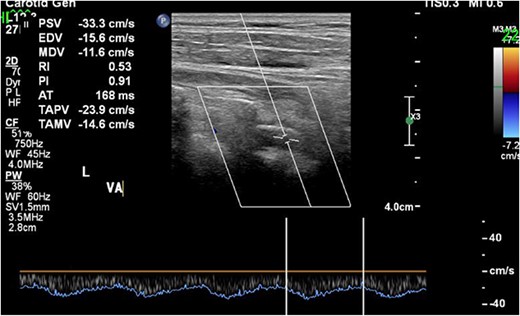
CDUS showed the antegrade flow of the left VA at the end of the operation. CDUS, carotid Doppler ultrasonic echography; VA, vertebral artery.
The post-operative course was uneventful, and the patient was discharged on the 16th day after the operation. The axillar-axillar bypass graft is patent, and there was no evidence of near syncope or vertigo during postoperative investigations (Fig. 6).
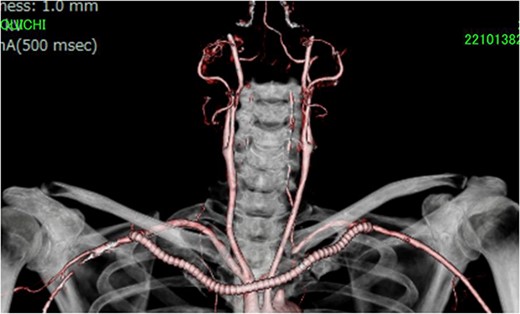
ECT after operation showed the patency of the bypass graft and adequate flow of the left VA. ECT, enhanced computed tomography; VA, vertebral artery.
DISCUSSION
Positional or rotational VBI causes vertigo, syncope or stroke, and is frequently associated with the VA compression. In contrast, SSS is typically detected by arm claudication or asymmetric arm blood pressure, but it can also be identified by vertigo or near syncope associated with head rotation [2]. Those mechanisms of pathogenesis are obviously different from each other.
Positional VBI is primarily caused by mechanical compression of the VA at the dominant extracranial course by cervical osteophytes or muscles. Therefore, the bloodstream of VA normally flows anterograde. In the SSS patients, the affected VA artery has weak, to-and-fro or retrograde flow from the contralateral VA through a circle of Willis. For that reason, any collateral arteries could not be detected and the steal phenomenon contributes to symptoms such as positional VBI.
In this case, CDUS assessment revealed Sakima grade type V retrograde VA flow [3, 4] and MRI angiography revealed hypoplasia of the right VA, which was not connected to the basilar artery, the right posterior cerebral artery, supplied by the right posterior communicating artery, and hypoplasia of the left posterior communicating artery. It was a rare condition without adequate blood supply from contralateral arteries. This kind of positional VBI would be caused by the VA compression as BHS or deterioration of the retrograde VA flow from collateral arteries of the internal carotid artery or muscle branches around the neck and shoulder [5]. Either way, SSS with BHS or collateral artery compression is a rare and undefined condition in this category.
Heidenreich et al. reported that untreated positional VBI are at risk of impending posterior circulation infarction [6]. Furthermore, more invasive examination for this type of positional VBI would be critical and intolerant because severe VBI could occur and weaken retrograde VA flow in the absence of contralateral artery supply.
In general, endovascular therapy for the long lesion had a high risk of stroke or embolisation due to occluded lesions measuring >3.0 cm. Furthermore, CT showed a possibility of left VA stenosis, but retrograde VA flow to the subclavian artery would indicate that it was not severely stenosed. As a result, axillary-axillary bypass surgery was performed as soon as possible to restore new sufficient blood flow to the left VA and the subclavian artery.
INVOS® was monitored during the operation to estimate the intracranial blood flow of the brain, and revealed no significant changes. Furthermore, the left VA was manually compressed for distal embolic protection on the timing of the anterograde recanalisation [7].
This report, of course, has limitations. First, due to a lack of any carotid ultrasonic echography experience, CDUS showed our original presentation and intra- and transcranial ultrasound assessments were not routinely documented at our institute. Second, for the same reason, the VA was unfortunately not assessed at the time of head rotation. Finally, there was no obvious angiogram of VA compression or collateral arteries compression.
In conclusion, we could describe a rare case of positional VBI with retrograde blood flow of the left VA. Vascular surgeons, in collaboration with neurological specialists, must assess the pathology of vertigo or syncope without preconception and should devise a strategy to maintain VA-patency with an incomplete circle of Willis after axillary–axillary bypass surgery [8, 9].
ACKNOWLEDGEMENTS
The authors would like to thank Enago (www.enago.jp) for the English language review.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
- presyncope
- ischemia
- magnetic resonance imaging
- syncope
- vascular flow
- cerebrovascular accident
- vertebrobasilar insufficiency
- vertebral artery
- ischemic stroke
- blood pressure
- axilla
- circle of willis
- subclavian steal syndrome
- surgical procedures, operative
- ultrasonics
- unconsciousness
- vertigo
- arm
- bypass
- computed tomographic angiography
- compression
- doppler ultrasound of carotid artery
- left subclavian artery
- fluid flow
- arm claudication



