-
PDF
- Split View
-
Views
-
Cite
Cite
Alex Mremi, Jack Bodganowics, Adnan Sadiq, Joshua Tadayo, Jay Lodhia, A giant metanephric adenoma in a young male, Journal of Surgical Case Reports, Volume 2023, Issue 4, April 2023, rjad187, https://doi.org/10.1093/jscr/rjad187
Close - Share Icon Share
Abstract
Metanephric adenoma is an uncommon renal tumor with almost exclusively benign behavior, which can clinically and radiologically imitate malignancy. The histological examination is therefore crucial in diagnosis. Herein, we report a case of an 18-year-old male with a huge left renal mass. Histopathology and immunohistochemistry of nephrectomy resection specimen confirmed it to be metanephric adenoma. We present our experience with this rare tumor entity and literature review with focusing on differential diagnosis.
INTRODUCTION
Metanephric adenoma (MA) of the kidney is a rare benign tumor with an incidence of 0.2–1%. They usually present as small renal mass and are treated by surgery (partial or radical nephrectomy) [1]. These tumors are commonly seen in adults between 50 and 60 years of age and rarely in the pediatric or younger groups. They are typically detected incidentally and they pose similar imaging features to Wilms’ tumor and papillary renal cell carcinoma [2]. Herein, we present an uncommon presentation of large metanephric adenoma in a young adult who was managed successfully.
CASE PRESENTATION
An 18-year-old male was referred to our center with gradual progressive abdominal distention for 1 year associated with on and off dull abdominal pain. He also reported passing blood-stained urine for 2 days which was painless. During the course of his illness, he also reported intermittent low-grade fevers and significant unintentional weight loss. He had normal diet with normal bowel habits and denied vomiting. On examination, he was undernourished with a body mass index of 17 kg/m2 with multiple bilateral inguinal lymphadenopathy. His axillary temperature was 36.9°C, saturating at 98% in room air with other vitals within normal range.
His abdomen was grossly distended and moving symmetrically with respirations. The umbilicus was inverted with no superficial veins. A huge nontender palpable mass extending from the left hypochondrium to the right iliac fossa crossing the midline was felt with dull on percussion over the mass and negative shifting dullness and fluid thrill. Bowel sounds were heard normal on auscultation. Other systems were essentially normal on examination.
His lab results showed a leukocyte count of 7.5 × 109/l, hemoglobin of 12.8 g/dl, normal platelet count of 299 × 109/l, serum urea of 3.67 mmol/l and creatinine of 67 mmol/l. Liver enzymes were within normal range. Urinalysis revealed mild proteinuria with severe hematuria. He underwent an abdominal-pelvic CT-scan that revealed a huge left renal tumor arising from the posterior cortex of the left kidney measuring 24 cm (AP) × 28.4 cm (T) × 31 cm (CC). The left kidney is displaced to the right lower quadrant. The mass crossed the midline with displacement of the aorta and pancreas to the left side. The small bowels were displaced inferiorly into the pelvis. There was no evidence of renal artery or vein thrombosis and no obvious lymph node enlargement seen (Fig. 1).
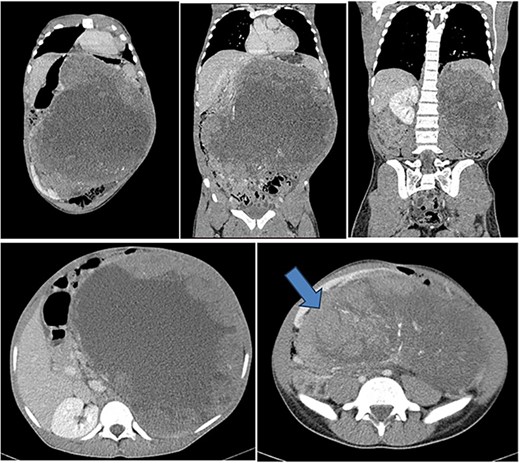
Contrasted coronal and axial CT of the abdomen and pelvis shows a huge heterogenous solid mass with central calcifications arising from the posterior cortex of the left kidney measuring 24 cm (AP) × 28.4 cm (T) × 31 cm (CC) in size. The left kidney is displaced to the right lower quadrant (blue arrow). The mass crosses the midline with displacement of the aorta and pancreas to the right side of the abdomen. The small bowels are displaced inferiorly into the pelvis. Features are suggestive of left renal tumor.
He was counseled on the planned procedure and consent was provided. Under general anesthesia in supine position, he underwent left nephrectomy successfully through a left transverse abdominal incision with no intra- and postoperative complications. The estimated blood loss was 300 mls; hence, no blood transfusion was given as he maintained normal blood pressure and pulse rate during and after the surgery. The specimen (Fig. 2) was submitted for histopathology analysis. Day 1 postoperative recovery in the intensive care unit was uneventful hence was transferred to the general urology ward, whereby oral sips were gradually initiated on day 2, abdominal drain removed on day 3 and was discharged on day 7. His follow-up clinic visits at weeks 2 and 4 were uneventful, respectively. Histology results showed a circumscribed hypercellular tumor composed of tightparked small to intermediate uniform, round cells with an embryonal appearance. These had hyperchromatic nuclei and scant cytoplasm. Areas with tubules or papillae were associated. Mitoses were rarely seen. Hemorrhage, necrosis and cystic changes were also noticed. Immunohistochemistry with epithelial membrane antigen (EMA) and cytokeratin-7 was all negative. These features were consistent with metanephric adenoma (Fig. 3).
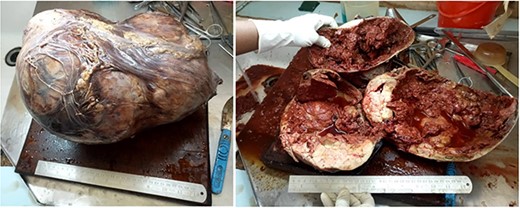
Photographs showing giant renal metanephric adenoma (specimen submitted post operatively). Radical nephrectomy specimen, irregular in shape measuring 35 × 25 × 25 cm. Cut sections revealed multifocal circumscribed solid tumor, brownish-white in color and the presence of thick brown cystic was seen. Extensive necrosis and hemorrhage were also associated along with remnant of normal appearing renal parenchymal tissue. No lymph node was identified.
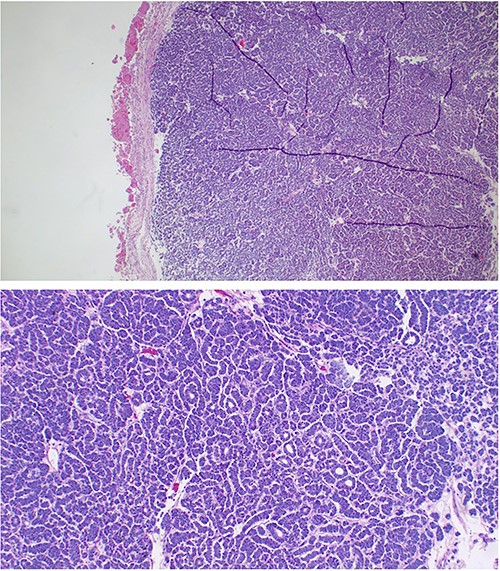
The sections showing circumscribed hypercellular tumor composed of tight parked small to intermediate cells. These were uniform round with an embryonal appearance. The nuclei were hyperchromatic with scanty cytoplasm. Tubular or acini structures were seen. Mitoses were rare. Hemorrhage and necrosis were also a feature. Immunohistochemistry with epithelial membrane antigen (EMA) and cytokeratin-7 was all negative. These features were consistent with metanephric adenoma.
DISCUSSION
MA of the kidney usually occurs in middle-aged individuals and twice as more common in females, contrary in our case the patient was rather young. They are usually asymptomatic hence diagnosed incidentally; however, signs and symptoms include hematuria, abdominal pain, hypertension and polycythemia [3]. In the index case similarly, he presented with abdominal pain most likely due to the compression effect of the mass and hematuria due to tumor necrosis. MA can occasionally be misdiagnosed as a malignant tumor due to the lack of specific clinical, radiographic and histological characteristics. Based on the occurrence of BRAF mutations, MA are said to be linked to the metanephric family of neoplasia, however, previously thought to be closely related to Wilms’ tumor and papillary renal cell carcinoma. In children especially, they can be difficult to distinguish from the abovementioned; however, metastasis has been reported in literature [4].
Generally, they are 3–6 cm but 15 cm diameter has been reported. However, the size of the mass was gigantic 30 cm in our case, making it an unusual presentation. Grossly they are well-circumscribed with a yellow surface as seen in Fig. 2. Histologically, they compose of acinar arrangement of small cells as seen in Fig. 2. Differential diagnosis includes Wilms’ tumor, renal papillary neoplasms and nephrogenic rests [5]. MA can be diagnosed using hematoxylin and eosin (H&E) staining, but immunohistochemistry is needed especially when samples are obtained by core needle. MA usually expressed WT1, CD57 and demonstrate focal CK7 in areas with elongated tubules [6]. 90% of MA also contain BRAFV600E mutations; BRAF mutations have also been identified in many other human malignancies including melanoma, papillary thyroid carcinoma and colonic adenocarcinoma [6, 7]. MA arises from renal medulla, whereas typical renal cell carcinoma arises from the renal cortex [7]. According to Zhu et al., MA are isodense or hypodense tumors without signs of hemorrhage on unenhanced CT-scan [8].
There are conflicting reports on the success of FNA to effectively diagnose MA. Since the natural history of these tumors is poorly known because they are resected upon diagnosis, radical or partial nephrectomy is suggested [9].
Metanephric adenoma of the kidney is a rare pathology which is worthy to report, especially with unusual presentation such as in our case with the gigantic size in a young adult. It can be confusing initially to yield a definitive diagnosis just from clinical and radiological investigations as it mimics renal malignancies therefore histology with immunohistochemistry confirms the diagnosis. Nonetheless prognosis is promising with surgery.
ACKNOWLEDGEMENTS
The authors would like to thank the patient for permission to share his medical information to be used for educational purposes and publication.
CONFLICT OF INTEREST STATEMENT
The authors report no conflicts of interest.
FUNDING
No funding or grant support.
AUTHORS’ CONTRIBUTIONS
A.M. and J.L. conceptualized and drafted the manuscript. J.B. was the lead surgeon. J.T. reviewed the medical records, A.M. prepared and presented the histology slides and A.S. reviewed and reported the radiology films. All authors have read and approved the final script.
INFORMED CONSENT
Written informed consent was obtained from the patient for publication for this case report; additionally, accompanying images have been censored to ensure that the patient cannot be identified. A copy of the consent is available on record.
DATA AVAILABILITY
No new data were generated or analysed in support of this research.



