-
PDF
- Split View
-
Views
-
Cite
Cite
Kentaro Kasa, Yuichiro Tanishima, Takanori Kurogochi, Takahiro Masuda, Fumiaki Yano, Ken Eto, Thoracoscopic repair of iatrogenic diaphragmatic hernia following multiple abdominal surgeries: a case report, Journal of Surgical Case Reports, Volume 2023, Issue 4, April 2023, rjad183, https://doi.org/10.1093/jscr/rjad183
Close - Share Icon Share
Abstract
Iatrogenic diaphragmatic hernia (IDH) is a rare complication that has been reported after various interventions, including liver transplantation, liver resection and nephrectomy. A surgical procedure for IDH has not been optimized. A 56-year-old man presented to our hospital with a 1-week history of abdominal pain and nausea. His medical history included an open nephrectomy for renal cancer 8 years ago and open distal pancreatectomy for its recurrence 1.5 years ago. Enhanced computed tomography showed IDH with the fornix of the stomach herniating to the left pleural cavity, without radiographic signs of strangulation. His symptoms improved after gastric decompression with nasogastric tube placement, and he underwent elective surgery. The incarcerated stomach was repositioned, and the hernia orifice was closed and reinforced with expanded polytetrafluoroethylene mesh using a thoracoscopic procedure. The patient had an uneventful postoperative course. The operative procedure for IDH should be tailored depending on anatomical alternations after previous surgeries.
INTRODUCTION
Acquired diaphragmatic hernia (DH) is mainly caused by trauma and rarely by iatrogenesis. Iatrogenic diaphragmatic hernia (IDH) has been reported to occur rarely after esophagus, liver and kidney surgery [1–6]. Surgical repair is required for symptomatic DH, but indications for asymptomatic DH remain unclear. Various methods for repair of IDH, e.g. transabdominal or transthoracic, with or without mesh) have been reported; however, the optimal surgical approach has not been established [7, 8]. Here we report a case of thoracoscopic repair for IDH after multiple abdominal surgeries, using expanded polytetrafluoroethylene (ePTFE) mesh.
CASE REPORT
The patient was a 56-year-old man with a history of hypertension, dyslipidemia, type 2 diabetes, hyperuricemia, hypothyroidism and left renal cancer. The patient underwent open left nephrectomy and adrenalectomy for renal cancer at 48 years of age, open distal pancreatectomy for local recurrence at 54 years of age and chemotherapy for metastatic lung cancer resulting in remission. The patient presented to our hospital with a 1-week history of abdominal pain and nausea. Physical examination revealed epigastric bloating without signs of peritoneal irritation. A chest X-ray showed gastric gas shadow upon the left diaphragm. Enhanced computed tomography (CT) showed the fornix of the stomach herniating into the left pleural cavity indicating a DH. There were no signs of gastric wall ischemia, necrosis or fluid collection (Fig. 1). Laboratory results showed only a mildly elevated C-reactive protein concentration (3.17 mg/dl) but otherwise were within normal limits. The stomach was decompressed after placement of a nasogastric tube, and the patient’s symptoms were improved. However, fluoroscopy revealed that DH was not reduced resulting in a poor gastric emptying indicating failure of conservative treatment. We semi-electively performed thoracoscopic surgery.
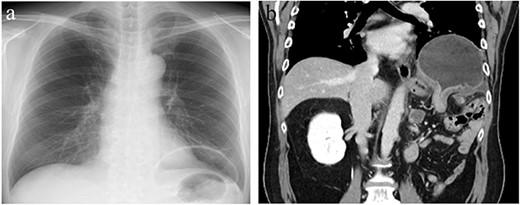
Chest X-ray (CXR) and contrast-enhanced computed tomography (CT). (a) CXR showing gastric gas shadow projected to left hemithorax. (b) CT showing a diaphragmatic hernia in the coronal plane.
Surgical procedure
The patient underwent thoracoscopic surgery in the right semi-lateral decubitus position under general anesthesia with two-lung ventilation. Four-port thoracoscopic surgery was performed for repair of DH with artificial pneumothorax (8 mmHg) (Fig. 2). The herniating stomach was easily repositioned back in the intraabdominal cavity. The size of the diaphragmatic defect was 40 × 30 mm (Fig. 3a and b). The hernial orifice was closed with 2-0 polypropylene sutures (Prolene, Ethicon, Raritan, NJ, USA) and reinforced with 90 × 60 mm ePTFE mesh (Gore DualMesh Biomaterial, W. L. Gore & Associates, Newark, DE, USA) (Fig. 3c and d). Intraoperative endoscopy showed mild edema of the stomach without signs of necrosis. The total operation time was 154 minutes, and total blood loss was minimal.
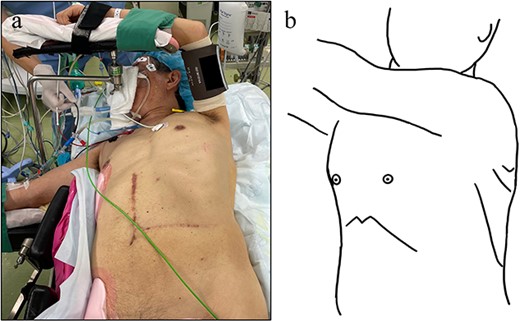
Surgical position and ports setting. (a) Right semi-lateral decubitus position. (b) Four ports in total. One 12-mm port at the left 4th intercostal space. Three 5 mm ports at the left fifth, sixth and eighth intercostal space.
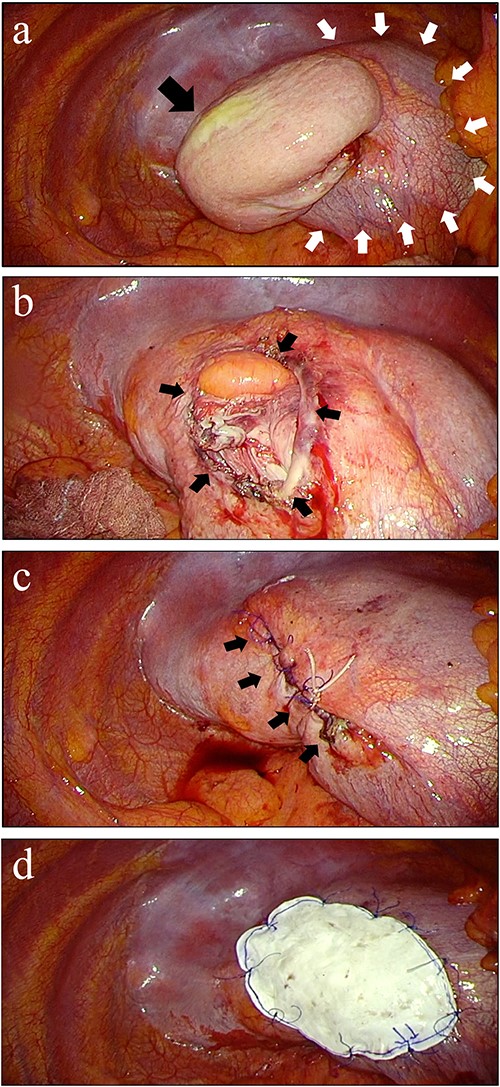
Thoracoscopic finding and repair of diaphragmatic hernia. (a) Incarcerated stomach (black arrow) from diaphragm (white arrows) was fornix without ischemia. (b) The defect on the diaphragm was 40 × 30 mm after adhesions was removed (black arrows). (c) Sewing the hernia defect with 2-0 polypropylene suture. (d) Reinforcement after hernia closure. An expanded polytetrafluoroethylene mesh sized 90 × 60 mm was fixed to cover the suture line and tendon part of the diaphragm.
Postoperative course
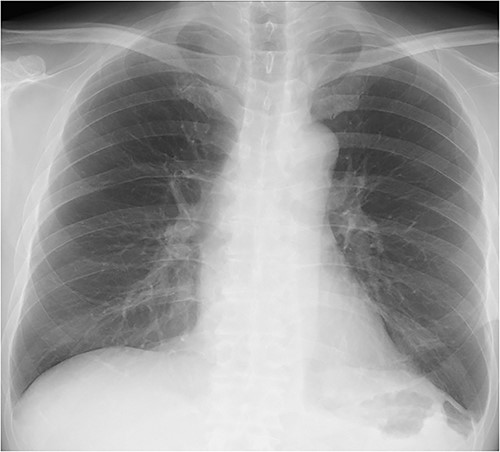
The patient’s postoperative course was uneventful. Oral intake was started the day after surgery, and the patient was discharged home on postoperative day 12. There was no sign of complication or relapse of hernia after surgery (Fig. 4).
DISCUSSION
Adult-onset DH is relatively rare and can be classified as congenital or acquired [9]. Acquired DH can be classified as traumatic or iatrogenic. Traumatic DH accounts for 0.8–6% of patients with blunt trauma and in more than 17% of patients with thoracoabdominal penetrating trauma [10]. IDH has been reported as a complication following esophageal hiatal hernia repair, liver transplantation, hepatectomy, radiofrequency ablation for hepatic tumor and nephrectomy [1–6]. The interval between primary surgery and incidence of IDH varies. Symptomatic DH of digestive tract organs, such as the stomach and the small intestine, requires surgical repair. The optimal timing and method of surgery are determined on a case-by-case basis. Although a small and asymptomatic Bochdalek hernia does not need to be treated surgically [11], there are no criteria to determine surgical indications for asymptomatic DH. A wait-and-see approach for asymptomatic DH is often recommended [2, 12].
Emergency surgical repair is needed if there are any signs of bowel ischemia or necrosis. Our patient was not required emergent surgery because (i) he did not have any laboratory and radiographic evidence of ischemia and (ii) his symptoms resolved after decompression with a nasogastric tube. Reduction of DH may not be expected without interventions because of the thoracoabdominal pressure gradient pushing the hernia contents into the thorax.
There is no ideal procedure of DH repair established; thoracoscopic vs laparoscopic, primary closure vs hernia patch and with mesh vs without mesh. A recent systematic review showed no difference in complication and recurrence rates of Morgagni hernia between thoracoscopic and laparoscopic surgery [13]. However, no study has compared surgical approaches for IDH. Hernia patch for IDH has not been reported. Primary closure with or without mesh reinforcement is usually applied [14]. Besides, no reports have compared DH repair with and without mesh, although mesh repair for DH seems to reduce the rate of recurrence [15].
Surgical repair for IDH is technically challenging due to adhesions secondary to prior surgery. In the case of bowel injury, mesh repair may not be preferred because of the risk of mesh infection. In our case, thoracoscopic approach was selected to avoid a higher risk of bowel injury in transabdominal approach. Positive intrathoracic pressure with artificial pneumothorax makes it easier to reposition the hernia’s contents into the intraperitoneal space. Mesh reinforcement is often applied for DH repair in both thoracoscopic and laparoscopic approaches [7]. The ePTFE mesh is often used for DH repair; however, the type of mesh is of no particular importance [14]. The mesh-to-defect area ratio (MDAR) is recommended to be at least 16:1 for the laparoscopic repair of ventral and incisional abdominal wall hernias [15]. However, the MDAR rule may not be applicable in the case of DH given a smaller area of the diaphragm limiting the size of mesh. In our case, the MDAR was 4.5:1.
Thoracoscopic surgery should be considered for treatment of IDH following multiple abdominal surgeries, in terms of avoiding intraperitoneal adhesions, providing a wider available surgical field for mesh application, and easier repositioning of the hernia contents with positive intrathoracic pressure. Thoracoscopic surgery for IDH is a useful and feasible approach for patients with a history of multiple abdominal surgeries.
ACKNOWLEDGEMENTS
We would like to thank Yoshio Tatsuoka for providing language support.
CONFLICT OF INTEREST STATEMENT
All authors have no conflict of interest regarding this paper.
FUNDING
This study was not funded.
DATA AVAILABILITY
The datasets supporting the conclusions of this article are included in this article.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This article is in accordance with the Declaration of Helsinki.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.



