-
PDF
- Split View
-
Views
-
Cite
Cite
Khushboo Singh, Ahmad Hlayhel, Sayali Kulkarni, Derick Christian, Case report: a rare case of gastroduodenal intussusception caused by GIST in an elderly patient and literature review, Journal of Surgical Case Reports, Volume 2023, Issue 4, April 2023, rjad055, https://doi.org/10.1093/jscr/rjad055
Close - Share Icon Share
Abstract
Gastroduodenal intussusception is a rare phenomenon in adults and is caused by gastrointestinal stromal tumors (GIST) in the majority of the documented cases. It commonly presents with abdominal pain, vomiting and melena. GIST is the most common gastrointestinal mesenchymal tumor in gastric and non-gastric locations. It is characterized classically by KIT or PGDFRA expression, and immunohistochemical analysis is the mainstay of diagnosis. Surgical resection provides definitive treatment in 70% of the cases. Here, we present a rare case of gastroduodenal intussusception caused by GIST in an elderly patient.
INTRODUCTION
Gastroduodenal intussusception in adults is a rare entity, found in only 10% of adult intussusception cases [1], as it typically requires a mobile structure, such as ileum, as an intussusceptum, and a relatively fixed structure, such as cecum, as an intussuscipiens [2]. A lead point pathology is responsible for about 80–90% of adult intussusceptions [3]. Here, we present a case of gastroduodenal intussusception caused by gastric gastrointestinal stromal tumor (GIST) in an elderly patient.
CASE REPORT
A 72-year-old patient with no past surgical history and a past medical history of developmental delay, hypertension and hyperlipidemia presented to the Emergency Department in mild distress with periumbilical abdominal pain, nausea and intractable vomiting for 1 day. The patient denied any hematemesis, bowel changes, fever, melena or hematochezia. On physical exam, the abdomen was unremarkable, and a computed tomography (CT) scan with contrast of abdomen and pelvis revealed a 5.6 × 5.3 cm heterogeneous mass arising from the body of the stomach extending into pylorus with no evidence of bowel obstruction and liver metastasis (Fig. 1). Subsequently, esophagogastroduodenoscopy (EGD) showed a large, fungating and ulcerated mass and it was reported to involve two-thirds of the luminal circumference in the gastric body (Fig. 2). Cold forceps biopsy showed gastric mucosa with hyperplastic changes, mild chronic active antral gastritis with focal intestinal metaplasia, negative for Helicobacter Pylori and dysplasia. Surgical management was discussed with the family, but they refused any surgical intervention.
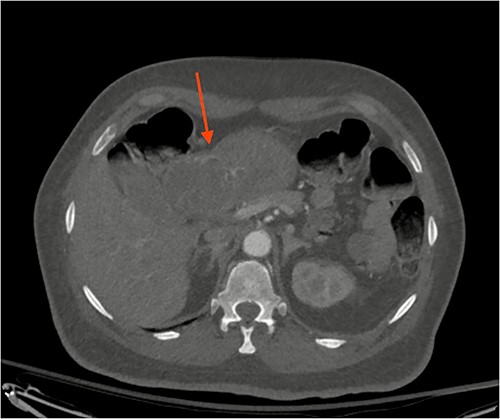
CT scan image of abdomen showing heterogenous gastric mass (red arrow).
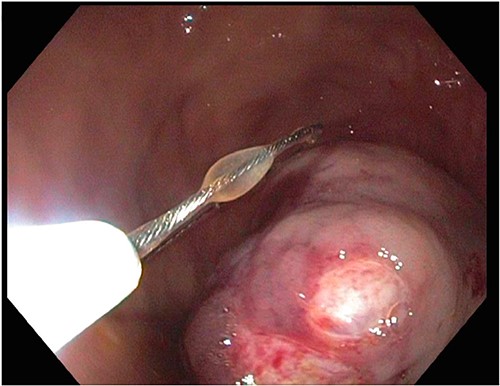
A large fungating gastric mass arising from the body of the stomach on endoscopy.
After a few days, the patient was found to have a large volume of melenic stool. A CT angiogram of the chest, abdomen and pelvis was done, revealing a mass in the antrum and pylorus of the stomach with a portion of the stomach pulled into it, consistent with gastroduodenal intussusception without any active extravasation. Repeat EGD showed a large, fungating, pedunculated mass with no bleeding and no stigmata of recent bleeding in the gastric body (Figs. 3 and4) and confirmed gastroduodenal intussusception. The intussusception spontaneously reduced while attempting to push passed the mass. The mass was too large to resect endoscopically. Given these new findings, the family agreed to surgical intervention. An exploratory laparotomy was made, and the patient underwent partial gastrectomy, distal antrectomy and Billroth II procedure. The resected large gastric body mass within the mid gastric body and two nodes were sent for frozen section and immunohistochemical analysis. The pathology report confirmed the mass as a GIST, spindle cell type with no significant nuclear atypia or mitosis, arising from muscularis propria, without mucosal invasion and R0 margins. The neoplastic cells stained positive for CD117 (KIT), CD34 and Desmin (focally). The tumor was T3N0M0 with a low mitotic rate, histologic grade G1 and was deemed low risk (3.6%). The patient was discharged to a subacute rehab with an uneventful recovery.
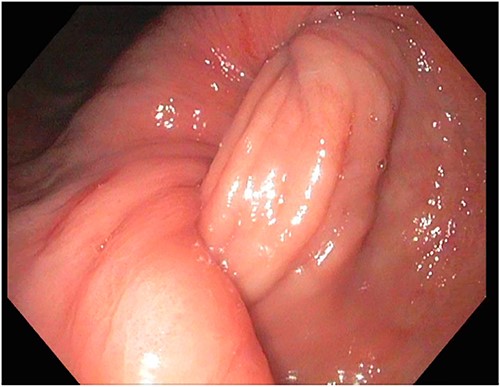
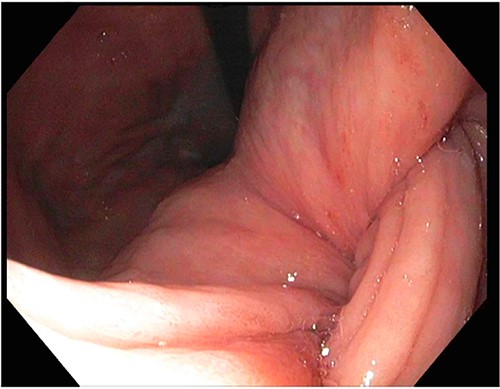
DISCUSSION
Most gastroduodenal intussusceptions present with acute epigastric pain, nausea, vomiting and melena, regardless of etiology. GIST is a common type of gastrointestinal mesenchymal tumor, and is commonly located in the stomach [4]. It only accounts for 1% of gastrointestinal tumors [5]. Molecular markers such as KIT, PDGFRA and DOG1 are the mainstay of providing the definitive diagnosis of GISTs [4, 6]. Zhang et al. [7] reported a similar case and compiled a list of 43 documented cases of gastric intussusception secondary to gastric tumors, out of which 24 were GIST. It is worth noting that it is responsible for >50% of the documented cases of gastroduodenal intussusception.
CT scan is the most accurate diagnostic imaging modality and detects the lead point [2]. In this case, CT scan confirmed the diagnosis of intussusception. Once diagnosed, the management in such cases is focused on the reduction of intussusception and resection of the tumor to prevent a recurrence. A preoperative diagnosis of tumor pathology is recommended to aid the decision of tumor resection and to plan chemotherapy. EGD can help attempt reduction, detect the bleeding source and obtain a biopsy. Endoscopic Ultrasound-Fine Needle Aspiration (EUS-FNA) is indicated for preoperative molecular diagnosis but can give poor diagnostic yield to assess mitotic rate [8]. If the EUS-FNA or EUS-Core biopsy is non-diagnostic, which is not uncommon, final diagnosis is made with surgical pathology.
Meanwhile, certain morphological features on CT scan provide vital clues toward the pathophysiology of the tumor, such as tumor size equal to or >5 cm and enlarged feeding vessels, irregular margins and intralesional hemorrhage are stipulated as the features of high-risk GISTs [9]. Hence, the location, size, enhancement and number of tumors on CT scan certainly assist the risk stratification and planning even in the absence of molecular diagnosis.
Generally, surgical resection is considered the standard of care in GIST cases. Often, resection of primary gastric GISTs can be done laparoscopically, but open, endoscopic and novel laparoscopic endoscopic cooperative surgery (LECS) can be employed [10]. Surgical management predominantly plays a role in (1) primary localized gastric or non-gastric tumors that are >2 cm to <10 cm, (2) stable disease, (3) limited progression and (4) emergencies [10, 11]. Tumors <2 cm can be monitored, and tumors up to 8 cm can be resected laparoscopically depending on tumor location [11]. The surgical technique is dictated by patient and tumor factors to achieve R0 or even R1 resection without disturbing the tumor pseudo capsule while preserving the function. In an antral GIST complicated by intussusception, antrectomy is preferable, which was done in this case [12]. Nodal metastasis is uncommon, and dissection is generally not required [11].
Although gastroduodenal intussusception in adults is rare, it can frequently present as a complication of GIST. There are various surgical approaches for the resection of tumors; however, partial gastrectomy may be preferable in some instances, and the decision should be made on a case-by-case basis.
DATA AVAILABILITY
The authors declare that data supporting the findings of this study are available within the article.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
CONFLICT OF INTEREST STATEMENT
None declared.



